Groundbreaking Model Unveils Secrets of Axonal Healing After Optic Tract Injuries
2025-03-28
Author: Ming
Introduction
A pioneering research team led by Prof. Liu Kai from the Hong Kong University of Science and Technology (HKUST) has introduced an innovative model for studying how optic tract injuries can heal through axonal rewiring. This new intracranial pre-olivary pretectal nucleus (OPN) optic tract injury model (pre-OPN OTI) sheds light on the complex mechanisms involved in functional axonal regeneration following central nervous system (CNS) injuries.
The Challenge of CNS Repair
In the adult mammalian CNS, the ability to repair itself after injuries is notoriously weak. Axons often fail to regenerate and reconnect with their target neurons, leading to significant functional impairments. Previous research has primarily focused on enhancing regeneration capabilities, but there have been limited models that investigate how functional connectivity can be achieved after complete injury. Many unanswered questions surround the mechanisms of functional circuit reconstruction in these circumstances.
Research Findings
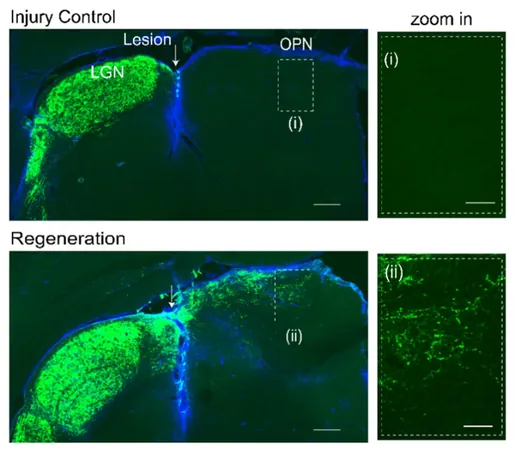
To address this critical gap, Prof. Liu's team published their findings in March 2025 in Nature Communications under the title “Functional optic tract rewiring via subtype- and target-specific axonal regeneration and presynaptic activity enhancement.” The pre-OPN OTI model employs advanced microsurgical techniques to apply mechanical pressure between the lateral geniculate nucleus (LGN) and the OPN, specifically targeting retinal ganglion cell (RGC) axons.
Advantages of the Pre-OPN OTI Model
This state-of-the-art model offers multiple benefits compared to traditional methodologies. By eliminating the need for cortical tissue removal, it simplifies surgical procedures and minimizes associated risks. The proximity of the injury site to the target nucleus (OPN) allows for focused studies on directed axonal regeneration. Moreover, the team utilizes the pupillary light reflex (PLR) as a quantitative measurement of functional recovery, along with maintaining high survival rates of RGCs post-injury for comprehensive long-term evaluation. This model represents a complete axonal injury scenario, with all functional recovery stemming exclusively from regenerated axons.
Gene Manipulation and Functional Recovery
The team's research revealed that knocking out the Pten/Socs3 genes in RGCs while expressing ciliary neurotrophic factor (CNTF) significantly promotes axonal regeneration towards the OPN and the restoration of functional synapses. Compelling evidence supports these findings, including super-resolution microscopy that demonstrated the colocalization of presynaptic and postsynaptic markers, along with electron microscopy that confirmed the formation of synaptic structures between the regenerated axons and OPN neurons. Additionally, trans-synaptic viral tracing and electrophysiological recordings validated the restored synaptic transmission and indicated partial recovery of the PLR, signaling successful functional reconnections.
Role of Intrinsically Photosensitive RGCs
Significantly, intrinsically photosensitive RGCs (ipRGCs) emerged as the crucial subtype responsible for mediating functional recovery, with regenerated axons accurately reconnecting to their original targets.
Dual-Intervention Strategy for Enhanced Recovery
To enhance the efficiency of axonal regeneration and functional recovery further, Prof. Liu's team proposed a strategic dual-intervention approach by combining axonal regeneration and synaptic enhancement. Through the knockdown of the lipid metabolism gene Lipin1 alongside the Pten/Socs3 knockout and CNTF expression, the rate of axonal regeneration was accelerated, reducing the PLR recovery time from six months to just three.
Optimizing Synaptic Transmission
Additionally, techniques such as overexpressing melanopsin to increase RGC photosensitivity and enhancing presynaptic voltage-gated calcium channel activity have been employed to optimize synaptic signal transmission, significantly improving overall functional outcomes.
Conclusion
This groundbreaking model not only revolutionizes our understanding of optic tract injuries but also provides a promising platform for future research aimed at restoring vision lost due to CNS damage. As researchers unveil the complexities of axonal rewiring, the potential for developing effective therapies to treat various neurological conditions may be closer than ever. Stay tuned for further insights from this vital area of study!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)