Groundbreaking Organoid Model Incorporates Microglia to Unravel Brain Inflammation Mechanisms
2025-04-03
Author: Sarah
In a stunning advancement for neuroscience, organoids have emerged as groundbreaking tools for disease modeling, drug testing, and developmental studies. Though they don't replicate human organs perfectly, these miniature systems provide invaluable insights into complex biological processes.
The Siegert research group at the renowned Institute of Science and Technology Austria (ISTA) has unveiled a novel organoid model that sheds light on how the developing nervous system responds to viral infections, such as Rubella. This revolutionary model promises to enhance pharmaceutical testing, especially in ensuring drug safety for expectant mothers.
Microglia are the brain's resident immune cells, acting like vigilant rangers in a vast forest. They monitor their environment for pathogens and initiate anti-inflammatory responses to combat them. Additionally, microglia are involved in maintaining a healthy balance of neurons and their connections, which is crucial for optimal brain functionality in adulthood.
Under the leadership of Sandra Siegert, the research team focuses on the crucial roles microglia play in communication with neurons during embryonic development. Their latest findings, published in the prestigious Journal of Neuroinflammation, detail a pioneering brain organoid model that incorporates microglia, allowing scientists to closely simulate inflammatory responses and test potential treatments.
Understanding Rubella's implications
Rubella, characterized by its distinctive rash, may seem like a harmless infection for children and adults. However, for pregnant women, the consequences can be dire. Classified among the TORCH infections, Rubella can be transmitted to the fetus, leading to profound developmental issues including brain malformations, which can increase the risk of schizophrenia in later life.
Unraveling the impact of viral infections like Rubella on brain development is essential, especially considering the troubling potential outcomes for the fetus. Ph.D. student Verena Schmied, together with Professor Sandra Siegert and their team at ISTA, is at the forefront of this critical research. They utilized retinal organoids—early models known for their distinct developmental pathways—to delve into these interactions.
3D Brain Models: A Closer Look
To create an organoid, researchers start with skin cells, which are reprogrammed into versatile human-induced pluripotent stem cells (hiPSCs). Under specific growth conditions, these hiPSCs cluster and mature into organoids that closely represent human brain tissue. Schmied emphasizes, "Retinal organoids uniquely recapitulate vital stages of early fetal brain development."
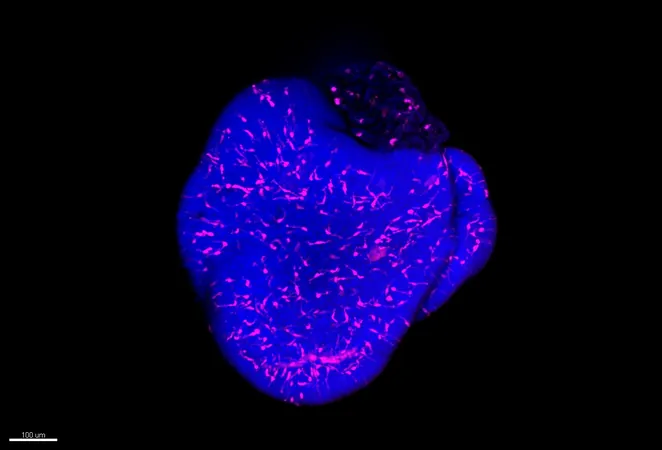
Previous organoid models for studying viral impacts didn't include microglia, key players in the brain's immune responses present from early developmental stages. Addressing this gap, the team successfully integrated microglia into their retinal organoids and confirmed their presence using advanced microscopy techniques. "It was astonishing to see microglia interact among the neurons in the organoid, visibly highlighted in pink against blue-stained neurons," recalls Siegert.
Impacts of Viral Infection on Neurodevelopment
The researchers examined how these organoids reacted to viral-like infections. By introducing a synthetic molecule that mimics viral components, they compared the reactions between organoids with and without microglia. The presence of microglia was found to trigger an inflammatory response, which led to excessive neuron division and potential disruption of neuronal circuitry—an alarming phenomenon that could result in neurodevelopmental disorders.
Siegert explains, "Microglia replicate the characteristics observed during inflammatory conditions, demonstrating the adverse consequences on the nervous system, which would remain unnoticed without them."
Exploring Treatment Options: The Ibuprofen Dilemma
While Rubella can be prevented through vaccination, effective antiviral treatments are lacking. Current approaches for managing symptoms often rely on anti-inflammatory medications like ibuprofen. The researchers assessed ibuprofen's effect on their organoids, particularly following viral infection. Remarkably, administering ibuprofen restored the normal neuronal environment, but only in the presence of microglia, indicating that the drug works effectively through microglial pathways.
"Our study underscores the necessity of incorporating microglia into brain organoid models for accurately mimicking inflammatory responses and exploring treatment effects. Their absence could lead to overlooking crucial therapeutic responses," Siegert asserts.
This groundbreaking research is particularly timely as it coincides with the increasing use of brain organoid models and hiPSCs in drug testing. It highlights the importance of creating realistic organoid systems, essential for advancing safety and efficacy studies in pharmacological research. The Siegert group's innovative model may pave the way for improved insights into brain development and safety mechanisms that protect both mothers and their developing children.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)