Groundbreaking Research Unveils Connection Between Mitochondrial Dysfunction and Cognitive-Metabolic Issues
2024-11-21
Author: John Tan
Recent Scientific Investigations
Recent scientific investigations have made a striking connection between mitochondrial dysfunction and cognitive-metabolic impairments. Mitochondria, often described as the powerhouses of the cell, contain their own unique DNA (mtDNA), which is crucial for cellular respiration and energy production. Unfortunately, mutations in this mtDNA can trigger a cascade of severe human diseases.
Research Initiatives
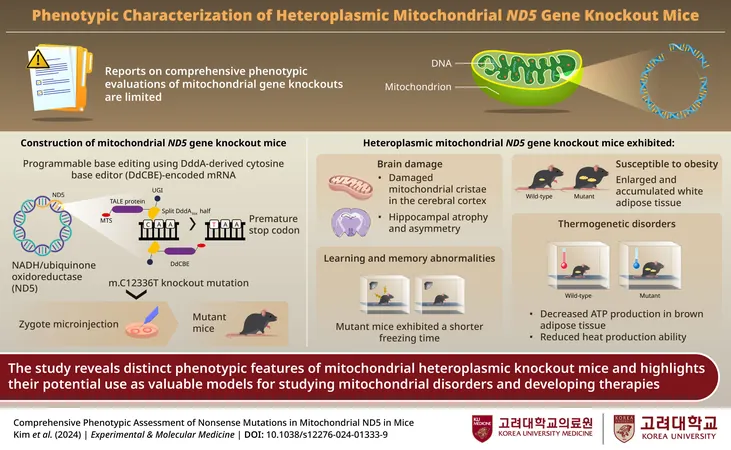
In a bid to deepen our understanding of mitochondrial genetic disorders, researchers from Korea have embarked on creating targeted animal models with specific mtDNA mutations. Despite previous efforts in this domain, the intricacies of phenotypic changes that occur due to mitochondrial gene knockouts—observable characteristics resulting from the inactivation of a specific gene—have remained largely unexplored.
Advanced Technology Application
To bridge this knowledge gap, a team of researchers led by Dr. Hyunji Lee, an Associate Professor at Korea University College of Medicine, utilized advanced programmable DNA base editing technology to investigate the effects of knocking out the ND5 mitochondrial gene. By inducing a nonsense mutation through the modification of a single nucleotide, the researchers introduced a premature stop codon in mice, which hinders protein synthesis, producing a truncated and generally nonfunctional protein, thereby leading to a loss of function.
Key Findings
The findings of this study, published on November 1, 2024, in the journal Experimental & Molecular Medicine, reveal that the knockdown of the ND5 gene results in reduced expression of multi-protein complex I and diminished ATP levels. Remarkably, the researchers observed significant morphological changes within the mitochondrial cristae of the cerebral cortex in these mice. There was also notable atrophy and asymmetry in the hippocampus, which correlates with cognitive deficits in learning and memory—evident through behavioral assessments showing slower movement and an impaired ability to recognize fear.
Metabolic Implications
The metabolic implications of mitochondrial dysfunction were also apparent, as researchers noted that the ND5 mutant mice exhibited increased susceptibility to obesity and thermogenesis disorders. These mutant mice struggled with proper body temperature regulation in cold environments, indicating a potential link between defective mitochondrial function and dysregulated fat tissue metabolism.
Clinical Ramifications and Future Directions
Prof. Lee emphasized the clinical ramifications of this research, asserting the need for mitochondrial-targeted therapies. He remarked, "Much like the pioneering gene editing treatment that received FDA approval last year, it is essential to see advancements in treatments utilizing mitochondrial gene editing technology for mitochondrial genetic diseases, which currently impact approximately 1 in 5,000 individuals globally."
Conclusion
Looking toward the future, this breakthrough sets the stage for innovative therapies targeting mitochondrial functions, which could drastically alter clinical approaches for managing prevalent health issues, including obesity and neurodegenerative disorders such as Parkinson's and Alzheimer's diseases. This study not only marks a significant stride in mitochondrial research but also offers hope for the millions affected by mitochondrial disorders worldwide. The future holds the promise of effective treatments that could improve the quality of life for those suffering from these life-altering conditions.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)