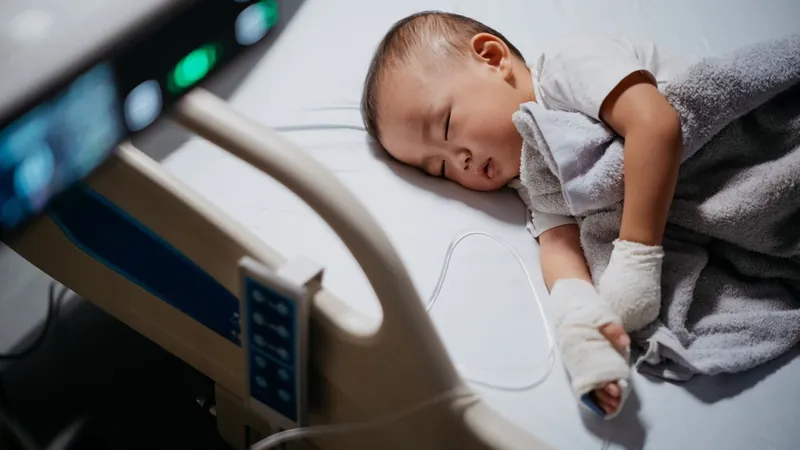
Groundbreaking RSV Drug Nirsevimab Shatters Records with Over 90% Effectiveness in Preventing Hospitalizations!
2024-12-09
Author: Rajesh
Introduction
A revolutionary new drug called nirsevimab (marketed as Beyfortus) has emerged as a game-changer in the fight against respiratory syncytial virus (RSV) among infants, boasting an impressive 93% effectiveness in preventing hospitalizations related to the viral infection, according to a recent study published in JAMA Pediatrics.
The Impact of RSV
As RSV is the leading cause of hospitalization for infants in the United States, this breakthrough comes as a beacon of hope. The findings indicate that nirsevimab not only significantly reduces the need for hospitalization but is also 89% effective at preventing various types of doctor visits due to RSV.
How Nirsevimab Works
Unlike traditional vaccines that stimulate the body's immune response to produce its own antibodies, nirsevimab offers infants a direct supply of lab-made antibodies to combat the virus.
Background on RSV Challenges
Before nirsevimab’s approval earlier this year, the medical community faced challenges in managing RSV, which affects 2 to 3 out of every 100 infants under six months, often leading to severe respiratory issues that require oxygen and intensive care. Symptoms typically start as mild as a runny nose or cough but can escalate quickly.
Study Data
Data from approximately 28,700 children under the age of five who sought medical attention during RSV season highlighted the profound need for effective prevention methods. Between the pre-approval period (2017-2020) and the recent 2023-2024 season, the researchers noted that out of the eligible infants, only a small number—402—actually received nirsevimab, a situation that could dramatically affect future RSV seasons if utilization increases.
Public Health Implications
Experts assert that with broader administration of this drug, we could witness a substantial public health impact, significantly reducing the number of RSV-related hospitalizations. The Centers for Disease Control and Prevention (CDC) currently recommends nirsevimab be administered to all infants under eight months old whose mothers have not received the maternal vaccine, ideally just before their first RSV season begins in October.
Challenges Ahead
However, challenges remain. The uptake of nirsevimab and the maternal vaccine has been sluggish this season due to supply issues and an unusually early onset of RSV in 2023. Nevertheless, the results from the study show undeniable promise for nirsevimab as an essential tool in safeguarding the health of our youngest and most vulnerable population.
Conclusion and Call to Action
With public health officials urging more widespread use, parents and caregivers are encouraged to stay informed and consult healthcare providers about nirsevimab for their infants as RSV season rolls on. This could potentially lead to a significant turning point in protecting babies from this dangerous virus.
Stay Tuned
Stay tuned for more updates on RSV treatments and strategies as the medical community continues to combat this pressing health concern!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)