Groundbreaking Study Reveals Critical Mechanism Behind Mitochondrial Division—Could It Unlock Secrets to Fighting Neurodegenerative Diseases?
2024-10-07
Author: Wei
Introduction
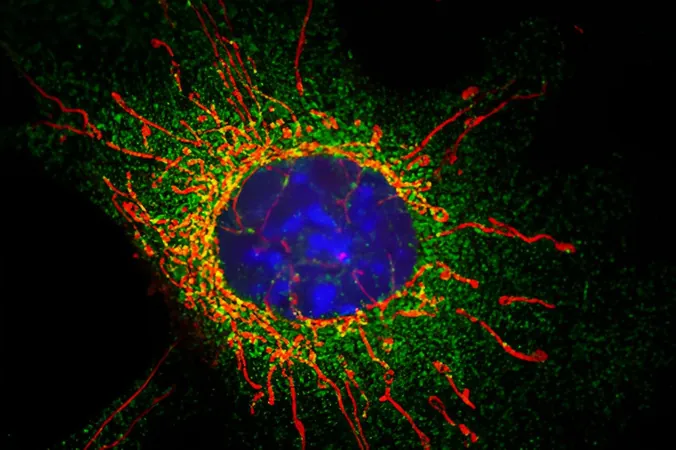
A revolutionary study led by researchers at the University of Bristol has unveiled a crucial molecular mechanism necessary for the division of damaged mitochondria, a process vital for maintaining cell health. This discovery holds significant implications for understanding mitochondrial dysfunction, which is a key factor in prevalent neurodegenerative diseases like Parkinson's and Alzheimer's.
Mitochondrial Functions
Published in the esteemed journal Science Advances, the research highlights the central role of mitochondria, often dubbed the "powerhouses of the cell," which generate the energy essential for cellular function. Beyond energy production, mitochondria also play critical roles in cellular signaling, metabolic regulation, and programmed cell death.
Key Findings of the Study
The study identifies the mitochondrial fission factor (MFF) as the pivotal protein that initiates mitochondrial division. MFF recruits another protein, known as DRP1, to facilitate this division. However, until now, the intricacies of this process were not entirely understood.
MFF and SUMOylation
In their groundbreaking findings, the research team revealed that MFF is subject to a specific modification known as SUMOylation, which is vital for its function. This modification allows MFF to disengage from inhibitory proteins that prevent division when mitochondria are damaged. Ultimately, this enables MFF to connect with DRP1 seamlessly and promote mitochondrial division—an essential process for cellular recovery.
Implications for Neurodegenerative Diseases
This discovery provides insight into why autosomal dominant mutations in MFF or related proteins can lead to pathological states characterized by fragmented mitochondria—a hallmark of many neurodegenerative diseases. Dr. Richard Seager, the study's first author, emphasized the importance of this finding, stating, "Our work highlights that the previous simplified model of mitochondrial division doesn't capture the complexity of the mechanism involved. Our new integrated model brings together various studies that were previously thought to be separate."
Expert Insights
Professor Jeremy Henley, a leading authority in Molecular Neuroscience at the University of Bristol and the study’s corresponding author, reiterated the critical role that mitochondrial dynamics play in overall cell health. "The misregulation of fusion and fission processes can lead to significant health issues, including the onset of severe neurodegenerative conditions," he noted. "Deciphering the molecular intricacies of mitochondrial division carries the potential for groundbreaking advancements in our approaches to prevent and treat these debilitating diseases."
Future Research Directions
The researchers primarily investigated this mechanism in non-neuronal cells, such as immortalized fibroblasts. However, they plan to expand their research to include neurons, where they will examine the impact of MFF-SUMO modification on mitochondrial shape and function, as well as its effects on neuronal connections—structures that heavily depend on the energy supplied by robust mitochondria.
Conclusion
As the scientific community moves forward, this study may lead to exciting new therapies that target mitochondrial dysfunction, offering hope in the battle against neurodegenerative diseases that affect millions globally. Stay tuned for more updates on this pivotal research that promises to illuminate the murky pathways of cell health and disease!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)