Groundbreaking Study Reveals How Histone Modifications Are Vital for Proper Blood Cell Formation
2024-12-18
Author: Sarah
Groundbreaking Study Reveals How Histone Modifications Are Vital for Proper Blood Cell Formation
In a pioneering study, researchers have unveiled the crucial role of histone modifications in regulating blood cell formation. A team led by molecular biologist Professor Gunnar Schotta from the Ludwig Maximilian University of Munich's Biomedical Center has shed light on the function of an enzyme known as SETDB1. This enzyme’s ability to epigenetically modify histone proteins plays a significant part in silencing specific DNA segments, ultimately influencing gene expression.
Every cell in our body carries the full genetic blueprint of an organism, but it is the chemical modifications on this DNA, referred to as epigenetic modifications, that dictate which genes are turned on or off under different conditions. The findings were published in the prestigious Proceedings of the National Academy of Sciences, showcasing the intricate mechanisms of gene regulation.
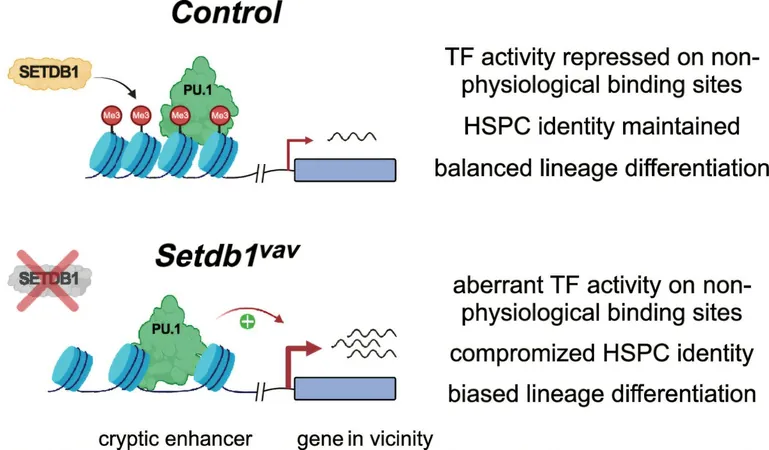
Professor Schotta emphasizes the focus of their research on endogenous retroviruses (ERVs)—DNA sequences inherited from retroviral insertions throughout evolution. Although these sequences are generally non-functional, they hold potential triggers for gene activation, acting as what are known as “cryptic enhancers.” When activated, these enhancers can significantly alter gene expression.
The study revealed that SETDB1 plays a pivotal role by adding a specific epigenetic marker, H3K9me3, to histones associated with these retroviral sequences. Interestingly, while this modification does not prevent transcription factors from binding to these sequences, it does inhibit their transcriptional activity. This suppression appears to be critical for maintaining normal gene expression levels.
Without the influence of SETDB1, the suppression of these cryptic enhancers fails, leading to dysfunctional gene expression patterns. Such abnormalities can severely disrupt the differentiation process of hematopoietic stem cells—the progenitors of all blood cells. As a result, there is an overproduction of myeloid and red blood cells, accompanied by a reduced capacity to form B and T immune cells, which are essential for a robust immune response.
This research not only advances our understanding of blood cell formation but also opens up new potential avenues for therapeutic interventions in blood disorders such as anemia and leukemia. By targeting the pathways affected by SETDB1 and the involved epigenetic mechanisms, future treatments may be developed to correct the imbalances in blood cell production.
Stay tuned as this exciting field of epigenetics continues to unfold, potentially revolutionizing our approach to treating a variety of blood-related diseases!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)