Groundbreaking Study Uncovers How Pancreatic Cells 'Remember' Cancer Signals Without Genetic Mutations!
2025-04-04
Author: Sarah
Introduction
In a revolutionary discovery by scientists at Johns Hopkins Medicine, researchers have unveiled that pancreatic cells can maintain a 'memory' of certain cancer-associated epigenetic marks, even in the absence of genetic mutations. This study, published in the journal *Genome Medicine*, adds a new layer to our understanding of how cancer develops, particularly in the context of pancreatic health.
Understanding Epigenetic Marks
Epigenetic marks are chemical modifications that influence gene expression without altering the DNA sequence itself. Think of it like the software of a computer that dictates how the hardware functions. Unlike mutations, which change the genetic code, these epigenetic modifications can suggest a pathway to cancer even before any mutations occur.
Research Findings
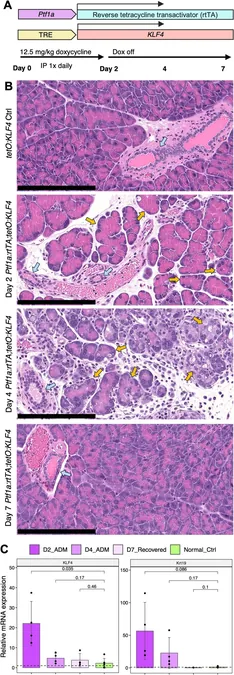
The researchers focused on the transition phases between normal pancreatic cells and cancerous ones in mice, revealing a significant pattern of these epigenetic marks during this crucial transition. Senior investigator Andrew Feinberg, M.D. from Johns Hopkins emphasized that cells can take on a hybrid identity due to factors like inflammation or damage, which can predispose them to cancerous changes without any direct genetic modifications.
The Role of Inflammation
Understanding this transformation is vital, particularly since inflammation has been recognized as a critical player in the onset of diseases, including cancer. In cases of pancreatic inflammation, acinar cells (which produce digestive enzymes) change into ductal cells (which transport digestive juices), a protective mechanism against the damaging effects of inflammation.
Genomic Sequencing Insights
In their extensive investigation, the team sequenced the genomes of mouse pancreatic cells undergoing a specific transition known as acinar-to-ductal metaplasia. Astonishingly, while they identified epigenetic changes on genes related to pancreatic cancer — notably within two critical groups of genes, PI3K and R/R/C GTPase — they found no accompanying mutations. This aligns with earlier findings in human precancers, suggesting that this unique 'epigenetic memory' allows normal cells to adopt characteristics of precancerous cells.
Persistence of Epigenetic Memory
Interestingly, when the transitioning cells reverted to their original acinar state, researchers observed that some of the cancer-linked epigenetic marks persisted for at least a week. This phenomenon indicates that cells can carry an epigenetic 'memory' of their cancer-like state, which Feinberg highlights as a significant factor in the cell's trajectory toward cancer.
Implications of the Discovery
This discovery also opens up a new conversation about why we might be seeing rising instances of cancers in younger populations, potentially linked to these epigenetic changes occurring without the need for age-related genetic mutations. The implications of this research could transform our understanding of cancer prevention and prompt new strategies for monitoring and treating pancreatic cancer.
Conclusion
As this field of epigenetics continues to unfold, the study not only provides hope but also emphasizes the complexity of cancer biology and the need for innovative approaches in fighting this formidable disease. Stay tuned, as further research is expected to delve deeper into these mechanisms, potentially leading us to breakthroughs in cancer therapies for years to come!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)