Groundbreaking Study Uses Atomic Force Microscopy to Uncover Microtubule Defects at Unprecedented Detail!
2024-12-12
Author: Sarah
Groundbreaking Study Uses Atomic Force Microscopy to Uncover Microtubule Defects at Unprecedented Detail!
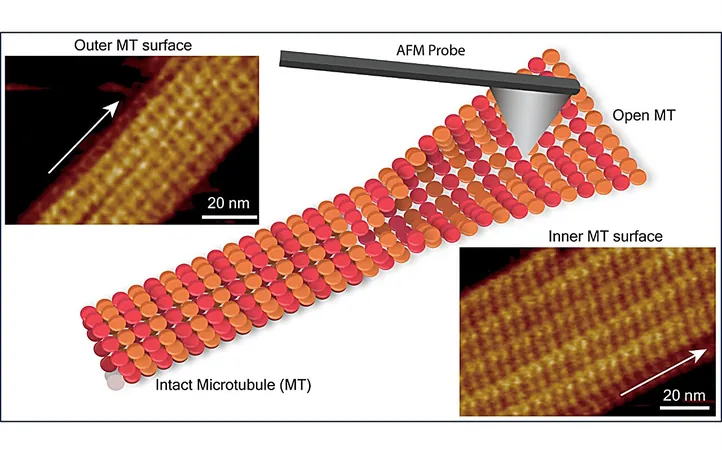
In an eye-opening study recently published in the journal *Nano Letters*, a team of researchers from the Nano Life Science Institute (WPI-NanoLSI) at Kanazawa University in Japan has made a significant breakthrough in understanding microtubules (MTs) by employing frequency-modulated atomic force microscopy (FM-AFM). This innovative approach has allowed them to visualize the submolecular structure of the inner surface of microtubules and detect structural defects within the microtubule lattice, offering essential insights into the complicated dynamic processes that govern microtubule function.
Importance of Microtubules
Microtubules are indispensable components of the cytoskeleton in eukaryotic cells, acting as scaffolds that are crucial for various cellular processes including cell division, cell migration, and intracellular transport. Composed of α-tubulin and β-tubulin proteins, these structures polymerize into dimers that assemble into linear protofilaments, forming a cylindrical lattice.
Limitations of Traditional Techniques
While traditional techniques such as X-ray crystallography and cryo-electron microscopy have provided valuable structural insights into microtubules, these methods often require complex sample preparation and extensive analysis, emphasizing the need for advanced techniques that can scrutinize MT's structural features and dynamics in real-time and under physiological conditions.
Gap in Research
Interestingly, prior research has extensively explored the outer surface of microtubules, but there has been limited focus on the submolecular arrangement of tubulin dimers on the inner wall. This study uniquely addresses that gap, considering that the inner and outer walls interact with different sets of proteins.
Research Team and Methods
Led by Ayhan Yurtsever, Hitoshi Asakawa, and Takeshi Fukuma, the research team utilized FM-AFM to delve into the arrangement of tubulin dimers on both the inner and outer surfaces of microtubules. Their findings revealed that while the inner MT surface presented a complex corrugated structure, the outer surface exhibited only shallow undulations. Notably, they discovered that one protofilament was considerably higher than its adjacent counterpart, a variation attributed to differences in the structural orientations and conformations of αβ-tubulin heterodimers.
Critical Findings
The study also identified a critical "seam" line on the inner surface believed to provide flexibility to microtubules. Additionally, FM-AFM enabled the detection of several lattice defects caused by missing tubulin subunits. These defects can dramatically alter the molecular arrangement of protofilaments, potentially impairing the functions of microtubules, which may have far-reaching implications in cellular biology.
Interaction with Taxol
Moreover, the research investigated how microtubules interact with Taxol, a chemotherapy drug that specifically binds to β-tubulin subunits within the αβ-tubulin dimers. By stabilizing microtubules, Taxol hinders cancer cell division and migration, thereby slowing down cancer progression. This binding action also serves as a marker, allowing for the differentiation of α- and β-tubulin subunits in high-resolution AFM images.
Conclusions and Future Directions
The insights gained from this groundbreaking study not only illuminate the complex architecture of microtubules but also underscore the potential of FM-AFM as a powerful tool for probing the molecular mechanisms of drugs that target microtubules. With continued research, this technology could pave the way for innovative cancer therapies and a deeper understanding of cellular dynamics.
Stay tuned for more exciting developments in the realm of nanoscience and cancer research, as this study is just the tip of the iceberg!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)