New Insights Unveiled: How Endogenous ZAP Influences Zika Virus Infections!
2024-11-09
Author: Arjun
Introduction
Recent research has revealed significant findings regarding the role of the zinc-finger antiviral protein (ZAP) in the infection phenotype of the Zika virus, highlighting its influence even in cell lines lacking a robust type I interferon response.
Methodology
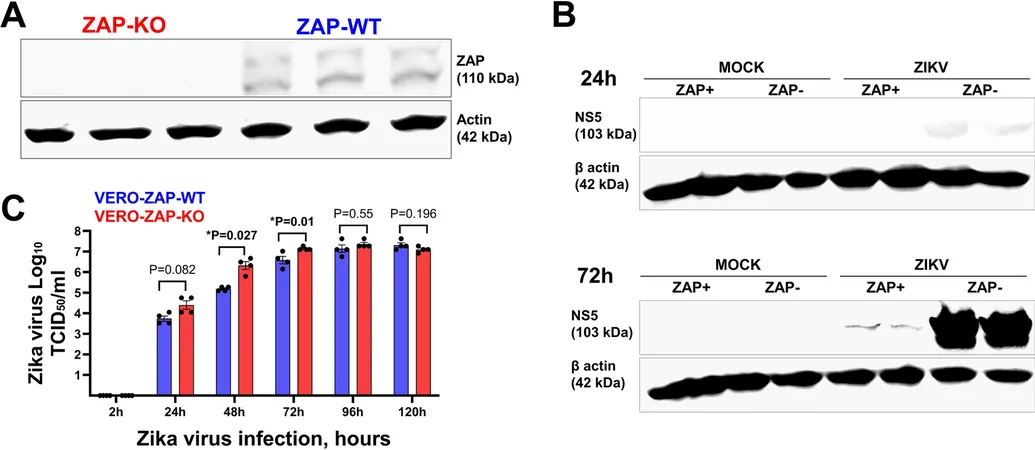
In this study, scientists utilized two cell lines—Vero-ZAP-WT (wild type) and Vero-ZAP-KO (knockout)—to investigate how ZAP impacts the expression of genes associated with Zika virus infection. The experiment involved seeding cells, inoculating them with Zika virus, and then extracting RNA to conduct RNA-seq analysis. This rigorous process included confirming viral infection using RT-qPCR and assessing RNA quality to ensure accurate results.
Key Findings
Key discoveries emerged: endogenous ZAP was linked to altered global gene expression patterns in both steady and infected states. Specifically, while fewer genes were differentially expressed in Vero-ZAP-WT cells during infection, ZAP-KO cells exhibited a notable surge in altered gene expression—342 genes at 72 hours post-infection compared to only 117 in their wild-type counterparts. This finding indicates ZAP’s critical role in modulating the cellular response during viral challenges.
Effect on Interferon Signaling
Intriguingly, the research also showcased ZAP’s impact on the interferon signaling pathway. It was observed that during Zika virus infection, both Vero-ZAP-WT and Vero-ZAP-KO cells displayed enhanced expression of several genes linked to immune responses, such as EGR1 and ISG15. This response appeared somewhat independent of the type I interferon signaling typically seen in competent immune cells.
Gene Set Enrichment Analysis
Furthermore, gene set enrichment analysis revealed that ZAP-WT cells presented greater enrichment in immune-related biological processes in contrast to ZAP-KO cells. Specifically, during Zika infection, ZAP-WT cells exhibited a significant increase in affected immune processes at the 72-hour mark.
Conclusion
The findings from this study not only reaffirm ZAP's role as a restriction factor for the Zika virus but also challenge previous assumptions regarding Zika's resistance to ZAP. Unlike earlier studies performed on various human cell lines, this research shed light on the complexity of ZAP functions in immune response dynamics, particularly in non-human primate Vero cells, which are known to lack certain immune signaling capabilities.
While the study was focused on a specific ZAP-KO clone, it serves as a groundbreaking step in understanding ZAP’s multifaceted role in antiviral responses, especially in non-interferon competent environments. The researchers emphasize the necessity for future studies to explore deeper interactions between ZAP, type III interferons, and Zika virus dynamics, noting that these insights could have far-reaching implications for antiviral strategies and therapeutic developments.
Future Directions
Stay tuned as we delve further into the fascinating world of antiviral proteins and their potential as crucial players in viral pathogenesis and immune escape!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)