Rare Genetic Mutation Uncovered as Key Factor in Devastating Neurological Disorder
2024-11-20
Author: Jia
Groundbreaking Study on Genetic Mutation and Neurological Disorder
A groundbreaking study has shed light on how a rare genetic mutation profoundly affects brain cell communication, offering pivotal insights into the root causes of a severe neurological condition known as developmental and epileptic encephalopathy (DEE). The research, released in the Journal of Neurochemistry, highlights a mutation in a regulatory calcium channel subunit that throws brain cell calcium handling and structural connections into disarray.
Research Background and Objectives
Conducted by a research team at Karl Landsteiner University of Health Sciences (KL Krems), this study informs our understanding of complex neurological disorders. The critical role of ion channels in the nervous system cannot be overstated, as they are essential for signal conduction, and the precise regulation of their function is necessary for neuronal communication.
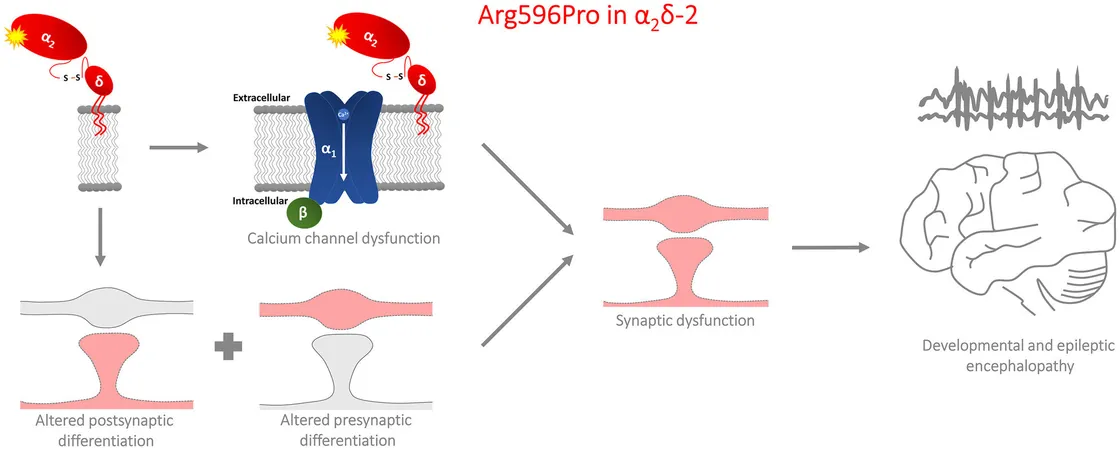
Focus on the α2δ-2 Protein
Researchers have been focusing on the α2δ-2 protein, known for its vital role in controlling calcium flow and organizing synaptic structures between neurons. The specific mutation studied, p.R593P, was observed in two siblings diagnosed with DEE. The analysis of this mutation reveals its detrimental effects on both calcium handling and synaptic organization within neurons.
Key Insights from the Research
Prof. Dr. Gerald Obermair, the lead investigator of the study, emphasizes the importance of these disruptions: 'This mutation alters the critical interplay between calcium channels and synaptic organization, essential for normal brain function. Our findings extend beyond DEE to potentially include a variety of neurodevelopmental and neuropsychiatric disorders linked to α2δ proteins.'
Mouse Model Findings
Employing a similar mutation in a mouse model, researchers examined the effects on hippocampal neurons—key players in learning and memory. Their findings showed that the p.R596P mutation considerably diminished α2δ-2's surface presence and synaptic localization, adversely affecting neuronal connectivity.
Identified Changes Due to the Mutation
The study identified three significant changes resulting from the mutation: 1. **Impaired GABAA Receptor Recruitment:** The mutation hampers the trans-synaptic recruitment of these receptors, crucial for inhibitory signaling in the brain. Poor inhibitory signaling leads to hyperactivity in neurons, a frequent cause of seizures in DEE patients. 2. **Altered Synapsin Clustering:** The mutation diminishes the clustering of synapsin, a vital protein for the structure of presynaptic excitatory, glutamatergic synapses. 3. **Reduced Synaptic Strength:** There is a decline in the amplitude of miniature postsynaptic currents, suggesting weakened synaptic connectivity.
Broader Significance of Findings
Sabrin Haddad, the first author of the study and a Ph.D. student, points out the broader significance of these findings: 'A single gene mutation can have extensive effects, impacting various aspects of neuron connectivity and ultimately leading to profound changes in brain function. Understanding these disrupted pathways could explain the severe neurological manifestations seen in DEE patients.'
Future Directions in Research
This research opens the door to exploring 'synaptopathies,' conditions related to disruptions in neuron connectivity. By revealing these intricate molecular mechanisms, this study not only deepens our comprehension of brain connectivity and development but may also pave the way for developing new treatments aimed at improving synaptic function and enhancing overall brain health.
Implications for Neurological Disorder Treatment
The implications of this study are vast, leading to a potential reevaluation of how we approach neurological disorders linked to genetic mutations, especially in the context of developing innovative therapeutic strategies.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)