Revolutionary 2D Metal-Organic Framework Detects Nitric Oxide with Unprecedented Sensitivity!
2024-12-12
Author: Ming
In our increasingly polluted world, detecting nitric oxide (NO) has become crucial for both environmental monitoring and medical diagnostics. This gas is notorious for its role in the combustion of fossil fuels, contributing to harmful effects like acid rain and smog. Moreover, in the medical field, NO serves as a vital messenger molecule, acting as a biomarker for conditions such as asthma.
Now, a groundbreaking international research collaboration has unveiled a new material that reversibly detects nitric oxide with remarkable sensitivity and selectivity while consuming minimal energy. This innovative solution comes in the form of a copper-based, electrically conductive, two-dimensional metal-organic framework (2D-cMOF), as detailed in the journal *Angewandte Chemie International Edition*.
Metal-organic frameworks (MOFs) are intricate lattice-like structures formed by metal "nodes" linked through organic bridges. Among the emerging categories of MOFs are electrically conductive ones that consist of stacked layers. These 2D-cMOFs have shown tremendous promise as chemiresistive sensors, which alter their electrical resistance when exposed to specific molecules, paving the way for sensitive and efficient detection of toxic gases.
However, previous iterations of such systems faced challenges, including cross-reactivity with various gases and limited reusability due to the irreversible binding of the target analytes.
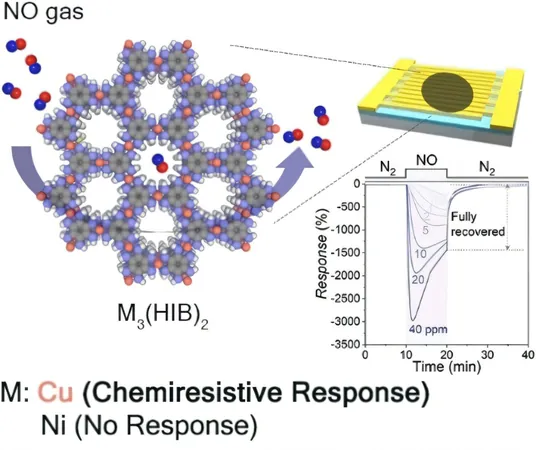
The brilliant minds at Dartmouth College (Hanover, NH, U.S.), the University of Oregon (Eugene, OR, U.S.), and Ulsan National Institute of Science and Technology (South Korea) have tackled these issues head-on. They developed a reusable 2D-cMOF specifically for the selective detection of NO, utilizing a framework made of copper and hexaiminobenzene, designated Cu3(HIB)2.
Their novel synthetic strategy involved introducing the linker as an undissolved powder into a solution of Cu2+ ions and potassium acetate. This approach significantly enhanced the material's crystallinity, yielding elongated rod-shaped crystallites measuring approximately 500 nm in length. The structure comprises stacked layers of six-membered rings interconnected by copper ions that bond to nitrogen atoms.
Advanced spectrometric analyses and computational studies revealed that the key binding sites for NO reside in the Cu-bis(iminobenzosemiquinone) units of the copper-2D-cMOFs. Interestingly, a similar compound created with nickel instead of copper exhibited negligible absorption of NO, underscoring the unique capabilities of the copper-based framework.
Crucially, the presence of single positively charged copper ions (in small quantities) in the structure plays a pivotal role in effectively binding NO. Theoretical models suggest that upon adsorption, the NO significantly distorts the framework's structure, causing destabilization of the bond. This distortion is essential, as it accounts for the material's remarkable ability to reversibly capture and release nitric oxide.
This groundbreaking work not only opens the door to improved air quality monitoring but also holds promise in medical diagnostics, potentially aiding in the management of respiratory conditions. With the capacity for high sensitivity and reusability, this innovative 2D-cMOF could revolutionize the way we approach the detection of harmful gases in both environmental science and healthcare. Stay tuned; the future of gas detection is here!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)