Revolutionary AI Techniques Transform ALS Research: New Hope for Patients
2024-12-20
Author: Li
Exciting developments in the field of amyotrophic lateral sclerosis (ALS) research have emerged from Keio University in Japan.
Led by neuroscientist Hideyuki Okano, PhD, a dedicated team has introduced an innovative method for cultivating functional lower motor neurons (LMNs) directly from induced pluripotent stem cells (iPSCs) obtained from ALS patients. This breakthrough not only holds promise for enhancing our understanding of the disease but also significantly accelerates drug screening efforts.
A Game-Changer in ALS Research
Dr. Satoru Morimoto, the corresponding author of the study, stated, “We aimed to develop a streamlined and robust approach that would accelerate ALS research and enable large-scale drug screening for this devastating disease, particularly in sporadic ALS patients.” With over 90% of ALS cases being sporadic, researchers have faced challenges due to the high variability among patient populations. Morimoto emphasized that analyzing a large sample size is essential to account for individual differences in disease expression.
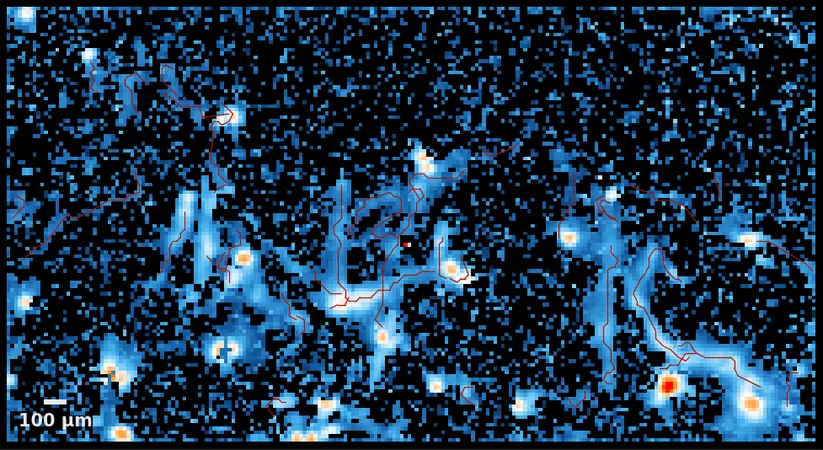
The team’s findings, published in *Stem Cell Reports*, detail a method that allows for an impressive induction efficiency of LMNs—reaching 80% within just two weeks, compared to conventional techniques. These LMNs successfully replicated ALS-specific features, such as the problematic aggregation of TDP-43 and FUS proteins. Using advanced multi-electrode arrays, they confirmed that these neurons exhibited firing and network activity analogues to mature neurons, providing a realistic model for further studies.
Leveraging AI for Precision and Efficiency
Crucial to this advancement is the integration of artificial intelligence (AI) in the research process. Morimoto acknowledged that achieving high induction efficiency while retaining purity of the cells posed a significant challenge. To tackle this, the team employed machine learning and AI-driven image analysis to enable live imaging, single-cell tracking, and reliable phenotype assessments, effectively filtering out non-LMN cells.
"This has streamlined a previously labor-intensive process, making ALS research more accessible and scalable," Morimoto noted, adding that automating data analysis has led to improvements in accuracy and the capacity to detect subtle cellular changes associated with disease progression.
The Future of ALS Treatment Could Be Bright
The implications of this research extend beyond ALS treatment alone. By creating a reliable platform for studying cellular vulnerabilities unique to ALS, this method opens up exciting possibilities for drug screening and personalized medicine. Morimoto is keen on pursuing three main goals: clarifying the mechanisms behind sporadic ALS, developing personalized drugs for individual patients, and utilizing iPSC-derived neurons as potential biomarkers.
Despite the promising advancements, Morimoto acknowledged that further real-time monitoring systems are required to reduce variability in results and enhance the robustness of these approaches. He expressed pride in the interdisciplinary efforts of his team, which has merged expertise across diverse fields—from stem cell biology to machine learning.
In conclusion, this remarkable study signifies a crucial step forward in ALS research, paving the way for better understanding, treatment strategies, and potentially transformative changes in the care of neurodegenerative diseases.
As Morimoto aptly stated, “We are committed to collaborating with others worldwide in our quest to find a cure for ALS.” Stay tuned, the future of ALS research looks incredibly promising!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)