Revolutionary Brain-Sensing CAR T-Cell Technology Promises Breakthroughs in Brain Tumor Treatment!
2024-12-11
Author: Rajesh
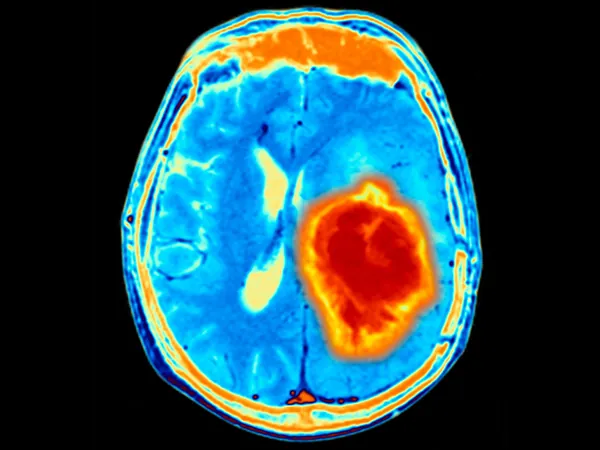
The Brain's Unique Challenge
Brain tumors, particularly glioblastoma, have long been regarded as one of the most formidable challenges in oncology due to the blood-brain barrier that complicates drug delivery and treatment. The UCSF team’s ingenious strategy involves programming T cells to identify distinct molecular markers present in the brain’s extracellular matrix—a complex environment that serves as a structural foundation for the organ’s cells.
Traditional cancer therapies often miss the mark by targeting single molecular markers, much like sending mail with only a street address. This can lead to adverse effects, as those markers may also appear in other tissues. In contrast, the UCSF team's sophisticated approach ensures targeted action by comparing it to sending mail with both a street address and a zip code. “With a living cell, this is what they typically do. They have instructions about where to go in the body and then look for molecular targets,” explained co-senior author Wendell Lim.
Cutting-Edge Mechanism: Brevican and Two-Factor Authentication
To implement this precision targeting, the researchers engineered synNotch receptors that activate upon detecting brevican, a protein found in abundance in the brain. Upon recognition, the T cells initiate a secondary program that activates a “killing receptor,” specifically designed to destroy tumor cells.
Lim likens this multi-factor approach to two-factor authentication: the T cells require two distinct signals to function—recognition of brevican (indicating their location within the brain) and identification of tumor-specific antigens. This dual requirement is crucial in preventing the T cells from mistakenly attacking healthy brain tissue. Remarkably, in experiments, even when tumors were implanted outside the brain, the engineered cells exclusively targeted and eliminated the tumors located within the brain.
Broadening Horizons: Beyond Glioblastoma
The implications of this technology extend far beyond glioblastoma treatment. The team demonstrated its potential to target breast cancer metastases in the brain, highlighting the versatility of the approach. “We can make a therapy based on this that attacks breast cancer metastases to the brain,” stated Lim, pointing to the adaptability of this system to various tumor types.
A New Era for Neuroinflammation and Autoimmune Disorders
Beyond its applications in tumor therapy, the "brain-sensing" capability of these engineered T cells opens exciting possibilities for tackling neuroinflammation and autoimmune diseases like multiple sclerosis. The researchers may not only eliminate brain tumors but also modulate the immune response in neuroinflammatory conditions, offering new hope for patients suffering from such diseases.
Promising Results and Future Trials
In preclinical trials, the engineered T cells exhibited impressive specificity and durability. One of the study's most striking results showed that previously treated mice could clear reinjected brain tumors almost immediately, indicating potential long-term immune memory. This feature could be a game-changer for cancer therapies, making treatments more robust over time.
Looking toward the future, UCSF’s team plans to transition into human trials, with the first clinical study expected to commence in 2025, initially focusing on glioblastoma and pediatric brain tumors. The modular design of the “zip code” system holds the promise for similar applications in other organs and diseases, opening a new frontier in cellular therapies.
As research progresses, this visionary technology could reshape the landscape of cancer treatment and beyond, reinforcing the hope that targeted cellular therapies can be the key to conquering some of the most challenging medical conditions.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)