Revolutionary Brain Tumor Research: Organoids Predict Response to CAR T Cell Therapy!
2024-12-09
Author: Sarah
Revolutionary Brain Tumor Research: Organoids Predict Response to CAR T Cell Therapy!
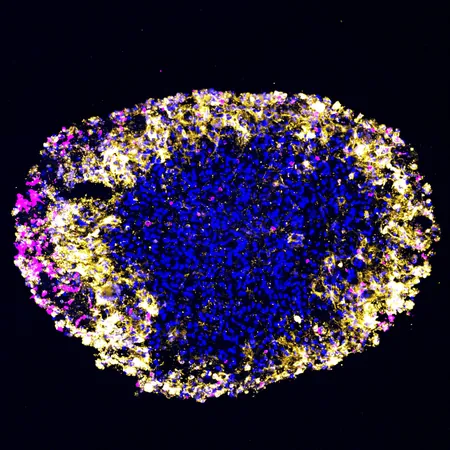
In a groundbreaking study, researchers at the Perelman School of Medicine, University of Pennsylvania, have successfully utilized lab-grown organoids from glioblastoma (GBM) tumors. This innovative approach allows for real-time modeling of patient responses to CAR T cell therapy, a critical advancement in personalized medicine for one of the most aggressive forms of brain cancer.
Harnessing the Power of Organoids
For the first time, scientists created organoids derived from actual patient tumors, enabling them to observe treatment responses that closely mirrored those in the patients' brains. As stated by Hongjun Song, Ph.D., one of the lead authors of the study, traditional methods for gauging response to therapy have been challenging. "We can't regularly biopsy the brain, and distinguishing between tumor growth and treatment-induced inflammation on MRIs can be tricky," he explained. These organoids provide a more precise reflection of an individual’s unique tumor biology, potentially revolutionizing treatment plans for GBM patients.
The Urgency of GBM Treatment
GBM is notorious for its aggressive nature, with patients typically facing a life expectancy of merely 12 to 18 months post-diagnosis. Despite extensive research efforts, effective treatments beyond surgery, radiation, and chemotherapy remain elusive. The complexities of GBM, which harbors various types of cancer cells and immune responses, complicate therapeutic approaches.
The Promise of CAR T Cell Therapy
CAR T cell therapy, which has gained FDA approval for certain blood cancers, involves reprogramming the patient's own T cells to target and obliterate tumor cells. Initial challenges in applying this therapy to solid tumors like GBM have motivated researchers to explore innovative strategies. Recent findings indicate that targeting two tumor-associated proteins instead of one may significantly enhance treatment efficacy for recurrent GBM.
A Collaborative Approach: Organoid and Clinical Trials
In the study, organoids were cultivated from tumor samples of six patients enrolled in a Phase I trial for a dual-target CAR T cell therapy. Remarkably, organoids can be produced within 2 to 3 weeks—allowing for timely analysis while patients recover from surgery. Following the treatment, researchers observed a correlation between the organoids' responses and the patients' tumor sizes via MRI, underscoring the potential of organoids to predict outcomes and guide therapeutic decisions.
Understanding Neurotoxicity Risks
One significant challenge of CAR T cell therapy is the risk of neurotoxicity, where treatment-related effects adversely impact the nervous system. In this research, immune cytokine levels—indicators of toxicity—were measured in both organoids and patient cerebrospinal fluid. Encouragingly, both exhibited similar trends, suggesting that organoids can serve as vital models for assessing the risk of adverse effects and enhancing treatment personalization.
A Bright Future for GBM Treatment
As Donald M. O'Rourke, MD, another co-senior author of the study, highlighted, these findings establish GBM organoids as invaluable tools for understanding the intricacies of brain tumor treatment with CAR T therapy. "Our vision extends beyond merely personalizing treatment; we aim to leverage these organoids to gain a deeper understanding of how to effectively combat this formidable cancer."
With this cutting-edge research, the hope is that patients will soon benefit from tailored approaches that significantly improve outcomes in the fight against glioblastoma, unlocking the potential for more effective therapies and improved quality of life. Stay tuned for further developments as scientists continue to harness the power of organoids in cancer treatment!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)