Revolutionary Breakthrough: Green Hydrogen Production Gets a Boost from Cage-Like Materials!
2025-04-22
Author: Arjun
Imagine a future powered by carbon-neutral hydrogen—yes, that future is closer than you think! Green hydrogen is rapidly emerging as a cornerstone of sustainable energy and a key ingredient for the chemical industry, produced via water electrolysis powered by renewable energy.
However, one significant hurdle remains: the oxygen evolution reaction (OER) during electrolysis tends to slow down hydrogen production. To unleash the full potential of hydrogen generation, scientists are racing to develop efficient catalysts for this process.
Unlocking the Potential of Clathrates
Enter Dr. Prashanth Menezes and his innovative team. They are diving into the world of clathrates—unique structures resembling cages that could revolutionize the way we produce green hydrogen. Traditional nickel compounds are already recognized for their cost-effectiveness and efficiency as catalysts. However, Menezes aims to explore whether clathrates, specifically those containing nickel, can supercharge the oxygen evolution reaction.
A Game-Changing Laboratory Collaboration
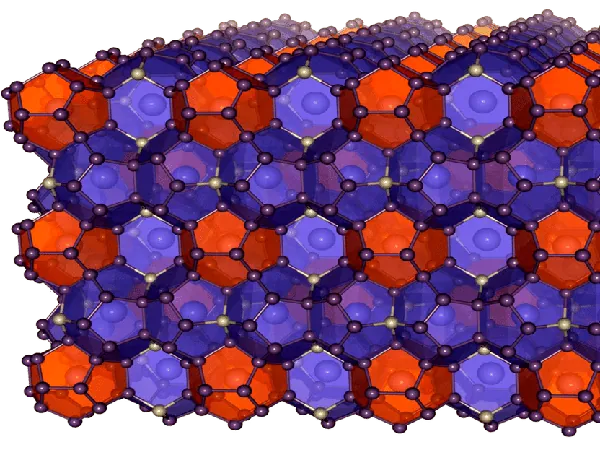
In collaboration with the Technical University of Munich, the team produced a groundbreaking material known as Ba8Ni6Ge40. This clathrate features a complex crystalline structure comprising polyhedral cages formed by germanium and nickel, encasing barium. Its extraordinary properties have made it a focal point for thermoelectrics and superconductors, but its potential as an electrocatalyst has remained largely untapped—until now.
Impressive Results from Cutting-Edge Experiments
Through rigorous electrochemical testing, the researchers discovered that Ba8Ni6Ge40 outperformed conventional nickel-based catalysts at a critical current density of 550 mA/cm², a benchmark for industrial applications. Even more astonishing, its stability held strong even after 10 consecutive days of operation!
Unveiling the Mystery: From Structure to Function
Using advanced techniques like X-ray absorption spectroscopy at BESSY II, the team delved deep into understanding why this clathrate performed so well as a catalyst. Their findings revealed that when immersed in an aqueous electrolyte and exposed to an electric field, the material undergoes a breathtaking transformation. The germanium and barium atoms dissolve, leaving behind a highly porous, sponge-like structure rich in nickel. This transformation greatly enhances the surface area, allowing more catalytically active nickel centers to engage with the electrolyte.
Dr. Niklas Hausmann, a member of Menezes' team, remarked, "This unexpected efficiency has opened our eyes to the potential of clathrates. We’re optimistic that similar results could be achieved with other transition metal clathrates, paving the way for a new frontier in electrocatalyst research!"
The journey towards fueling the world with sustainable, green hydrogen is just getting started, but with breakthroughs like these, it's only a matter of time before we see a cleaner, greener future!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)