Revolutionary Breakthrough in Blood Cancer Treatment: Teaching Immune Cells to Target Mutant Stem Cells!
2024-10-02
Author: Mei
Recent Breakthrough in Blood Cancer Treatment
Recent groundbreaking research led by Dr. Leonard Zon at Boston Children's Hospital has revealed a promising new strategy for combating blood cancers, including leukemia. The study focuses on manipulating blood stem cells, which are crucial to our circulatory health and are responsible for producing various blood cell types, by enlisting the help of immune cells known as macrophages.
Role of Macrophages in Quality Control
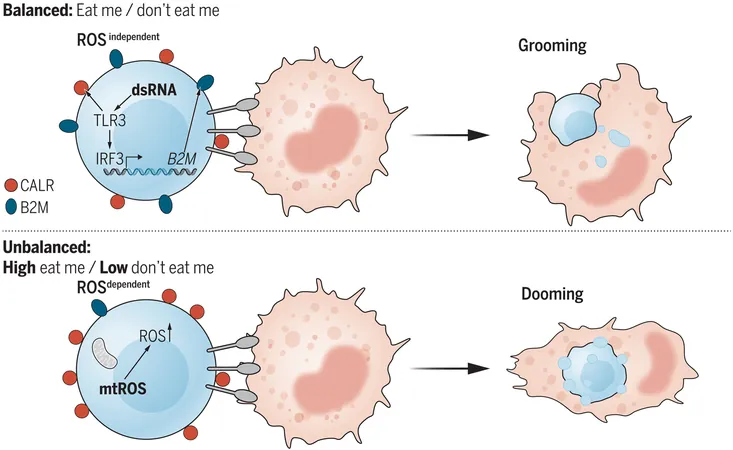
During their development, blood stem cells must pass through a rigorous quality assurance protocol, where macrophages act like vigilant quality controllers. They identify and eliminate any diseased or mutated stem cells flagged by unique surface molecules, such as calreticulin. This molecule functions as an “eat-me” signal that instructs macrophages to engulf unhealthy cells, all while promoting the proliferation of healthy stem cells.
Discoveries from Zebrafish Experiments
By conducting experiments on zebrafish, Zon’s lab was able to demonstrate that calreticulin can be stimulated through various means. More intriguingly, researchers discovered a counterpart signal—termed beta-2-microglobulin (B2m)—which serves as a 'don't-eat-me' signal. This two-way signaling mechanism opens a window into potential treatment strategies for blood cancers.
Published Findings in *Science*
In their published findings in the journal Science, Zon explained, “We aim to train macrophages to effectively eradicate mutant stem cells.” While macrophages can remove these errant cells occasionally, their efficacy wanes, leaving room for cancerous clones to procreate and dominate. This research hints at a revolutionary approach to enhancing blood cancer treatment.
Identification of Key Compounds
To identify the origins of the “eat-me” signals, Zon and his team screened over 1,200 chemicals, ultimately isolating 93 compounds that prompted calreticulin production. Notably, more than half of these compounds appeared to spike cellular levels of reactive oxygen species (ROS), while others did so through alternative pathways, demonstrating that control over these mechanisms could significantly affect stem cell fate.
Innovative Use of CRISPR Screening
Further contributions to this study include the innovative use of whole-genome CRISPR screening of human leukemic cells to pinpoint more than 10,000 genes that regulate the macrophages' response to stem cells. The exploration suggested that an active 'don't-eat-me' signal exists, prominently featuring the TLR3 gene, which plays a vital role in innate immunity.
Key Insights on Macrophage Functionality
Key insights revealed that macrophages were able to dispose of blood stem cells more efficiently when TLR3 was knocked out. The absence of B2m led to an uptick in blood stem cells being engulfed and destroyed, showing the enormous potential of manipulating these signaling pathways for future therapies.
Next Steps in Research
The Zon Lab's next steps include a rigorous search for drugs capable of enhancing the 'eat-me' signal specifically in mutant stem cells. This precision could herald a new era of treatment for preleukemia and leukemia, with possibilities extended beyond cancer treatment to tackle clonal hematopoiesis—a condition that can signal higher risks for cancer as people age.
Optimism for Future Implications
Dr. Zon is optimistic about the future implications of this research, stating, 'By effectively modifying these signals, we're looking at a way to eliminate harmful stem cell clones while preserving healthy ones. This strategy could significantly reduce the risk of developing cancer in the future.'
Conclusion and Future Outlook
With the potential to transform the landscape of blood cancer treatment, this research not only holds promise for patients battling current conditions but also offers innovative preventive measures to halt cancer before it begins. Keep an eye on these developments—they could soon reshape the future of oncology!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)