Revolutionary Breakthrough in Gut Microbiome Engineering!
2024-12-12
Author: Arjun
Introduction
A dynamic team of researchers from Carnegie Science, led by Will Ludington, Karina Gutiérrez-García, and Kevin Aumiller, has made groundbreaking strides in our understanding of the gut microbiome. Their recent publication in *Science* unveils crucial genes that empower beneficial bacterial species to thrive in specific gastrointestinal regions, potentially transforming the future of microbiome engineering.
The Importance of the Gut Microbiome
The gut microbiome consists of a diverse community of microbial species residing within our bodies, significantly influencing health, fertility, and even longevity. These microbial populations are integral to various functions, including digestion, immune responses, and pathogen defense, illustrating their vital role in maintaining overall well-being.
Microbiome Distribution in the GI Tract
Interestingly, the microbiome is not uniformly distributed along the gastrointestinal tract. Each section of the digestive system serves distinct functions in processing food and absorbing nutrients, mirroring the specialized roles of different microbial communities. Consequently, successful colonization by diverse bacterial populations hinges on several factors, including nutrient needs, local pH levels, oxygen content, competition with other bacteria, and the harsh conditions posed by stomach acid and bile salts.
Understanding Bacterial Navigation
As explained by Ludington, "We are dealing with an incredibly complex system of interconnected microbial communities. Each species must find its ideal location to thrive and enhance the health of its host." Researchers have been tirelessly investigating how specific bacterial species navigate to their respective sites and how undesirable strains are kept at bay.
Analogies to Airport Luggage Handling
Drawing an analogy to checked luggage in a bustling airport, Ludington likened the gut's microbial community management to an efficient baggage handling system that ensures each bag reaches its designated destination. "In the gut, beneficial bacteria, much like luggage, need to settle in the right area to establish a flourishing colony,” Gutiérrez-García noted.
Role of Adhesins in Colonization
The research team identified that the success of colonization largely depends on specific proteins in bacterial cell walls known as adhesins, which allow bacteria to adhere to varied surfaces within the body. However, the challenge lies in understanding how these helpful microbes find their precise locations.
Innovative Research Methods
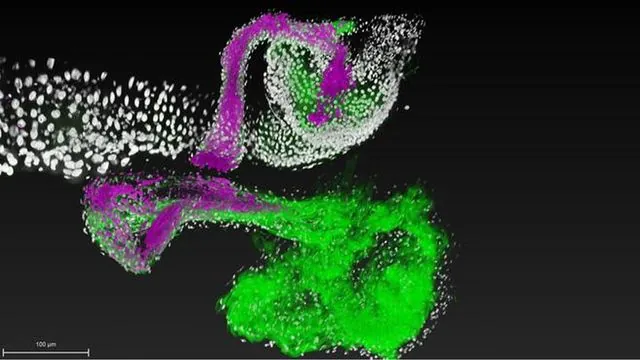
To investigate this phenomenon, the team employed cutting-edge technology that allowed them to observe a single cell of the bacterial species *Lactiplantibacillus plantarum* colonizing the gut of fruit flies in real-time. This innovative method, developed in collaboration with scientists from renowned institutions such as Johns Hopkins University and UCLA, has provided unprecedented detail into how individual bacterial cells interact with their host gut.
Findings on Bacterial Attachment
The fruit fly, a common laboratory subject, proved to be an ideal model organism due to its well-characterized and limited microbiome composition. By meticulously tracking the interactions, the researchers discovered that *L. plantarum* obtained from wild fruit flies could establish stable attachments to host tissues, while strains sourced from humans only achieved transient connections.
Genetic Underpinnings of Microbial Relationships
Armed with this insight, the team undertook the arduous task of pinpointing the genetic underpinnings of this remarkable affinity for gut colonization. Their findings revealed a set of genes responsible for establishing enduring microbial relationships within specific niches.
Implications for Probiotic Engineering
"This research provides critical insights that pave the way for engineering bacteria tailored to particular needs in the human gut," asserted Aumiller, one of the study's co-lead authors. "We are now equipped to develop probiotics that could be optimized for specific areas of the digestive system."
Future Directions in Research
The excitement doesn’t stop there! Ludington and his team are now focused on unraveling the mechanisms that dictate these binding preferences, forging a path toward a more profound understanding of microbial dynamics.
Conclusion
This revolutionary study not only advances our knowledge of gut microbiome functionality but also holds immense potential for future therapeutic applications, such as probiotics engineered for optimal health benefits. Stay tuned as researchers unlock more secrets behind the enigmatic world of gut bacteria!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)