Revolutionary Breakthrough: Scientists Create Almost Perfect Water-Repellent Material!
2024-12-12
Author: Wei
Revolutionary Breakthrough: Scientists Create Almost Perfect Water-Repellent Material!
In an exciting development that could transform various industries, scientists from the Karlsruhe Institute of Technology (KIT) in Germany and the Indian Institute of Technology Guwahati (IITG) have engineered a groundbreaking surface material that exhibits nearly total water repellency. This innovative material utilizes metal-organic frameworks (MOFs), which are highly porous structures created from metal ions interconnected with organic linkers, reimagining their potential by attaching hydrocarbon chains.
The resulting material showcases superhydrophobic properties—meaning it can repel water droplets so effectively that they form nearly perfect beads on its surface. This advancement paves the way for revolutionary applications in self-cleaning surfaces for automobiles, building materials, and various other fields that demand resilience against moisture and environmental wear. The details of this research have been published in the cutting-edge journal, Materials Horizons.
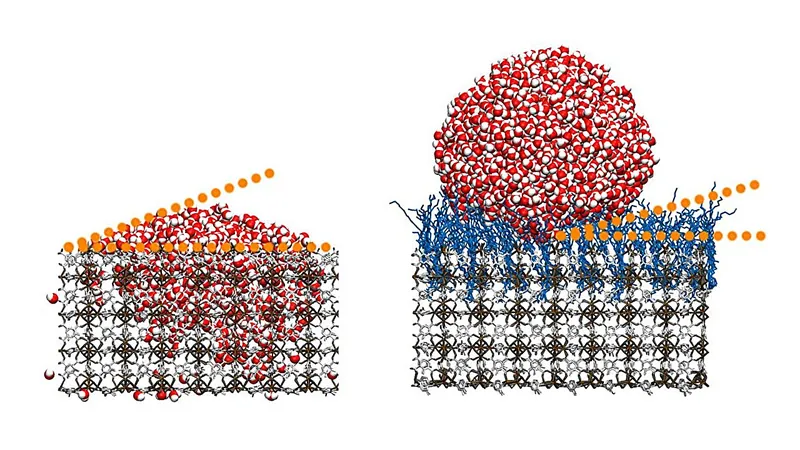
MOFs are already renowned for their unique characteristics; just two grams of the substance can cover an area equivalent to a football field. This incredible volumetric potential has made MOFs a focus for applications in gas storage, separating carbon dioxide, and even new medical technologies. However, the research team capitalized on the outer surfaces of these crystalline materials by enhancing them with hydrocarbon chains, leading to astonishing findings.
Professor Christof Wöll, from KIT's Institute of Functional Interfaces, explained, "Our method enables us to create superhydrophobic surfaces with contact angles exceeding 160 degrees. This is a substantial leap beyond what is achieved with currently available smooth surfaces and coatings." It’s important to note that while previous studies attempted to explore the wetting properties of MOF powders, the application of monolithic MOF thin films in this context represents a pioneering concept.
The study's impressive outcomes are attributed to the unique arrangement of the hydrocarbon chains, which adopt a disordered, coil-like shape—a phenomenon known as a “high-entropy state”. This exceptional structure is critical for the material's hydrophobic capabilities and is not replicable in other substances. Remarkably, the research revealed that introducing perfluorinated hydrocarbons—typically known for their superhydrophobic qualities—reduced water contact angle instead of increasing it. Observations showed that these perfluorinated molecules did not achieve the energetic conditions necessary for the high-entropy state, crucial for maximizing water repellency.
The team also experimented with surface roughness on a nanoscale, strategically manipulating the physical characteristics to diminish water adhesion further. Their findings showed that even at slight angles, water droplets would effortlessly roll away, significantly enhancing both the hydrophobic and self-cleaning properties of the material.
Professor Uttam Manna from IITG's Chemistry department remarked, “Our comprehensive theoretical analysis connects the experimental anomalies to the high-entropy state of the molecules grafted onto the MOF films. This research is poised to revolutionize the design and production of next-generation materials with unparalleled hydrophobic properties.
This advancement in material science not only opens doors to enhanced self-cleaning technologies but also sheds light on the potential for reducing maintenance and cleaning costs in various industries. As researchers continue to explore the vast implications of this work, one can only imagine the impact this superhydrophobic material will have on our everyday lives in the near future!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)