Revolutionary Catalyst Paves the Way for Climate-Neutral Methane Production!
2024-10-04
Introduction
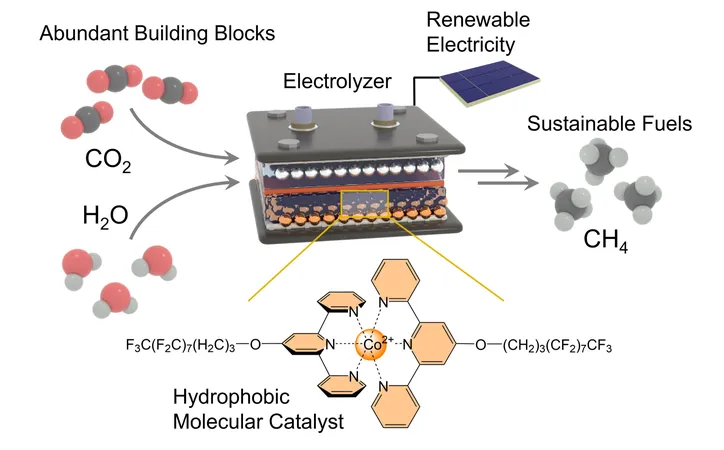
In a groundbreaking study, researchers from the University of Bonn and the University of Montreal have unveiled an innovative catalyst that produces methane from carbon dioxide (CO2) and water (H2O) with remarkable efficiency using electricity. This remarkable achievement invites a new era in energy production, particularly as methane, a key component of natural gas, serves crucial functions—ranging from heating homes to acting as a primary ingredient in the chemical industry.
Climate-Neutral Methane Production
What makes this development even more intriguing is that when methane is generated using renewable energy sources, it can be considered largely climate-neutral, representing a significant stride in combating climate change. The ramifications of this study extend far beyond methane production; the processes and insights gleaned could be leveraged to synthesize other essential chemical compounds in the future. The findings were recently published in the prestigious journal *Nature Chemistry*, generating excitement throughout the scientific community.
Sustainable Approach to Methane Synthesis
Traditional chemical reactions often rely on heat or pressure to initiate, but this team opted for a more sustainable alternative—electricity. According to Prof. Dr. Nikolay Kornienko, the lead researcher who recently transitioned from Montreal to Bonn, "By harnessing eco-friendly electricity, we have the ability to produce methane that does not exacerbate global warming."
The Challenges of Methane Production
The process of synthesizing methane—chemical formula CH4—is no walk in the park, particularly due to the complexity of executing a reaction between gas and liquid components. The research team devised a clever solution by utilizing a gas diffusion electrode, allowing them to combine CO2 and H2O effectively. This crucial reaction involves separating oxygen from the carbon atom and substituting it with hydrogen atoms sourced from water.
A Battle Against Competing Reactions
However, challenges abound—in this case, water tends to engage in a competing reaction that leads to the formation of hydrogen and oxygen when an electric current is applied. "If we allow this auxiliary reaction to take place, our methane production halts,” warned Morgan McKee, a key assistant in the research. "Our challenge is to prevent water from making contact with the electrode while still employing it in the reaction."
The Role of the Catalyst
This is where the newly minted catalyst shines. Coated onto the electrode, it promotes the swift reaction of carbon dioxide while simultaneously inhibiting the unwanted side reactions that could derail production. By utilizing an "active center," the catalyst facilitates the detachment of oxygen atoms from carbon and enables faster hydrogen integration—a game changer in methane synthesis.
Hydrophobic Innovation: A Smart Solution
The catalyst not only works to expedite the reaction but also relies on specially designed long molecular side chains that repel water. "These hydrophobic chains keep water molecules at bay from the active center and the electrode, while effectively collecting hydrogen atoms from the surrounding water to channel them directly to the carbon atom," explained Prof. Kornienko, also affiliated with the Transdisciplinary Research Area "Matter" at the University of Bonn.
Future Prospects
The impressive efficiency rate of over 80% and the minimal presence of undesirable byproducts underline the significance of this research; however, the current catalyst is not yet optimized for large-scale methane production. Kornienko remains optimistic, stating, "Though our catalyst isn't ready for commercial deployment, the principles we pioneered can indeed be adapted to develop more effective catalysts suitable for industrial applications."
Conclusion
Looking to the future, Kornienko believes that this revolutionary technique could extend beyond methane, with potential applications in producing other valuable chemical compounds such as ethylene, which is crucial for numerous plastic products. This innovative research not only holds promise for a more sustainable future but might just reshape how we approach energy and chemical production, pushing us one step closer to climate neutrality. Stay tuned as we continue to follow this fascinating development in the world of green technology!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)