Revolutionary Discovery: How Compact Nucleoli Could Unlock the Secret to Anti-Aging
2024-11-25
Author: Arjun
Recent groundbreaking research from Weill Cornell Medicine has unveiled a potentially game-changing insight into the biology of aging, suggesting that maintaining the nucleolus—a critical structure within cell nuclei—compact could be key to fighting the aging process. The study, published in *Nature Aging* on November 25, reveals that the size of the nucleolus plays a crucial role in cellular longevity, and its findings could pave the way for new longevity therapies.
The researchers observed these phenomena in yeast—a surprisingly fitting model organism that shares cellular characteristics with humans. As individuals age, they become increasingly susceptible to diseases like cancer, cardiovascular issues, and neurodegenerative disorders, all of which are linked to cellular aging. Dr. Jessica Tyler, a leading expert on the study, emphasized, “Aging is the highest risk factor for these diseases. Instead of targeting each illness individually, we should aim to delay the onset of these diseases by addressing the root molecular issues at play.”
The Role of the Nucleolus
The nucleolus is responsible for producing ribosomal RNA (rRNA), essential for protein synthesis, and houses ribosomal DNA (rDNA). This area of the genome is particularly vulnerable due to its repetitive sequences, making it difficult to repair if damaged. Such damage can lead to severe consequences, including chromosomal alterations and eventual cell death.
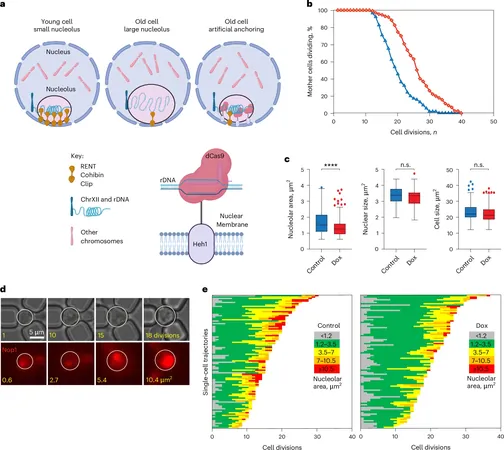
Notably, the size of nucleoli tends to increase with age across various organisms, from yeast to humans. However, lifestyle interventions such as calorie restriction have been shown to keep nucleoli smaller and may contribute to increased longevity. Dr. Tyler and her colleague, Dr. J. Ignacio Gutierrez, sought to further explore the impact of nucleolar size on aging. They devised a novel technique to anchor rDNA to the nuclear membrane in yeast cells, allowing them to manipulate nucleolar size independently.
Key Findings: The Mortality Timer
Their experiments revealed that compact nucleoli significantly slowed aging—offering results comparable to those achieved through calorie restriction. However, what stood out was the non-linear growth pattern of nucleoli as cells aged. For the majority of their lifespan, cells maintained smaller nucleoli, but upon reaching a certain threshold, nucleoli would expand rapidly, indicating a shift towards cellular mortality. This size explosion marked the beginning of a countdown to the cell's decline, with studies showing that cells usually survived only five additional divisions after surpassing this critical threshold.
The researchers noted that larger nucleoli exhibited less stable rDNA and became permeable to proteins that typically wouldn’t enter. This influx of potentially harmful factors can lead to instability within the genome, ultimately accelerating the aging process. “Condensates like nucleoli play critical roles in keeping biological reactions efficient by isolating them,” explained Dr. Tyler. When this compartmentalization fails, it can result in catastrophic genomic consequences.
Next Steps: From Yeast to Humans
The investigative team plans to extend their research to human stem cells, which are vital in replacing damaged or dying cells. Although stem cells display remarkable regenerative potential, they too have a lifespan and eventually cease to divide. Researchers hope to translate their findings on nucleolar size and its role in aging to enhance the longevity of human stem cells, potentially transforming the landscape of regenerative medicine.
With these promising findings, the quest for longevity treatments that target molecular aging may soon take a significant leap forward. Stay tuned for more updates, as the implications of this research could put traditional anti-aging approaches on the backburner forever!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)