Revolutionary Discovery in Bacterial DNA Enzyme Could Pave the Way for New Antibiotics
2024-11-25
Author: Sarah
A groundbreaking study by researchers from Durham University, Jagiellonian University in Poland, and the John Innes Center has unveiled significant insights into DNA gyrase, a crucial enzyme found in bacteria and a primary target for antibiotic development. This enzyme, essential for bacterial DNA supercoiling, is absent in humans, making it an ideal target for designing antibiotics that can selectively disrupt bacterial function without harming human cells.
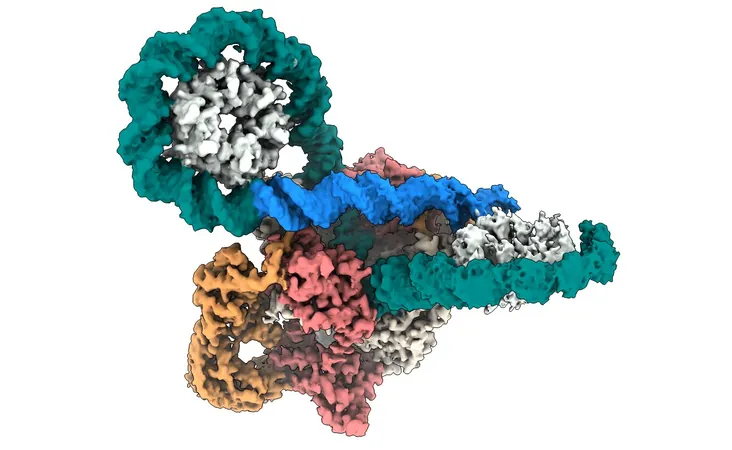
Leveraging high-resolution cryo-electron microscopy, the team has provided an unprecedented view of how DNA gyrase interacts with DNA. This discovery could signal a significant leap forward in the fight against antibiotic-resistant bacteria, a growing global health crisis that necessitates urgent innovation in antibiotic therapies.
The study, recently published in the Proceedings of the National Academy of Sciences, details how DNA gyrase operates as a complex molecular machine. It carefully twists and stabilizes DNA, much like winding a rubber band; however, unlike a rubber band that unwinds when released, DNA gyrase secures the twisted form, enabling bacteria to thrive.
Crucially, the enzyme employs a unique 'figure-of-eight' looping mechanism that allows it to break and reconnect DNA strands. This meticulous process is vital—should the DNA remain unsealed, it would result in the death of the bacterial cell. Current antibiotics, particularly fluoroquinolones, exploit this weakness by inhibiting the resealing action of gyrase, ultimately leading to bacterial cell death. However, the rise of antibiotic resistance highlights the necessity for a deeper understanding of this enzyme's mechanics to design better therapies.
The researchers’ innovative use of cryo-electron microscopy produced detailed imagery of gyrase during its action on DNA, contradicting many long-held assumptions about how this enzyme functions. Professor Jonathan Heddle from Durham University commented on the implications of these findings, stating, "Our results challenge previous notions of the order and position of gyrase's complex moving parts during supercoiling, which could significantly influence how we approach the design of new inhibitors."
This study not only furthers our comprehension of bacterial biology but also opens exciting pathways toward developing antibiotics that can effectively target gyrase while navigating the challenges posed by antibiotic resistance. The researchers plan to capture additional detailed snapshots of gyrase at various stages of its action, which will help create a complete molecular narrative of its operational process.
With this advanced understanding, there is a promising outlook for the creation of next-generation antibiotics that are not only more effective but also specifically engineered to combat bacterial infections resilient to existing treatments. The implications for public health are immense, potentially changing the landscape of antibiotic therapy in the ongoing battle against infectious diseases.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)