Revolutionary Discovery Sheds Light on Huntington's Disease and Opens Doors for New Treatments!
2025-01-08
Author: Ming
Groundbreaking Study Overview
In a groundbreaking study led by researchers at the University of California, Irvine (UCI), a revolutionary discovery has unveiled intricate molecular mechanisms linked to Huntington's disease (HD), providing new potential targets for treatment. This landmark research not only deepens our understanding of Huntington's but also establishes connections with other devastating neurodegenerative diseases including amyotrophic lateral sclerosis (ALS), frontotemporal lobar dementia (FTLD), and Alzheimer's disease.
Study Insights
The study, published in the prestigious journal *Nature Neuroscience*, sheds light on the complex effects of the genetic mutation responsible for HD—a repeat expansion of cytosine, adenine, and guanine nucleotides. While the mutation's existence is known, how it disrupts cellular functions has remained a perplexing mystery.
Key Molecular Mechanisms
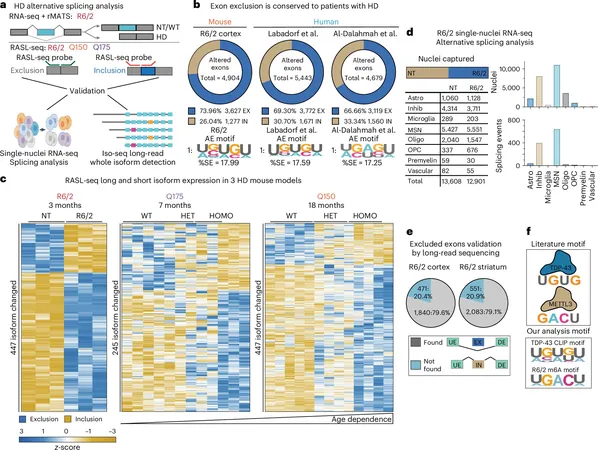
The researchers identified key players in the RNA processing defects inherent to HD: the RNA-binding protein TDP-43 and the m6A RNA modification, a chemical tag that influences RNA stability and function. Astonishingly, the binding interactions between these two regulators were found to be altered in the genes associated with HD. Notably, TDP-43 pathology, traditionally linked to ALS and FTLD, was also detected in the brains of individuals with Huntington’s disease.
Future Treatment Possibilities
“Our findings herald a new era of potential treatments," stated co-corresponding author Leslie Thompson, Ph.D. "By probing deeper into the roles of TDP-43 and m6A modifications, we unveil their promise as therapeutic targets that could resonate across various neurological conditions.”
Technological Advances in Research
Drugs optimized to interact with these molecular pathways could revolutionize our approach to neurodegeneration, perhaps even offering a glimmer of hope for treatment in HD and other diseases characterized by TDP-43 dysregulation. Thompson emphasized the significance of the research, which employs clinically relevant models to unravel novel RNA-based mechanisms that contribute to aberrant gene regulation.
Research Methodology
Under the leadership of UCI assistant project scientist Thai B. Nguyen, the team employed cutting-edge genomic and molecular biology techniques to investigate how m6A modifications guide TDP-43's regulatory role. Notably, the research utilized critical tissue samples from global brain banks, amplifying our understanding of the essential processes governing RNA splicing.
Findings and Implications
The study uncovered a concerning trend: in both HD mouse models and human patients, TDP-43 mislocalization and m6A RNA modification disruptions were found to impair TDP-43's binding to RNA. This dysfunction culminates in significant RNA processing irregularities, particularly affecting the striatum—a brain region acutely involved in Huntington’s pathology.
Expert Opinions
"This research is groundbreaking,” stated co-corresponding author Robert Spitale, Ph.D. “By focusing on key processes like RNA modification and splicing, we not only enhance our comprehension of the molecular disruptions driving HD but also pave the path for prospective therapeutic interventions that could span a range of neurodegenerative disorders.”
Conclusion and Future Directions
As scientists continue to explore these promising findings, the hope remains that future clinical applications might mitigate the devastating effects of Huntington's disease and its related disorders. Stay tuned as this important field of research unfolds and potentially transforms the future of neurodegenerative disease treatment!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)