Revolutionary Fixed-Dose Tablet Offers Hope in the Fight Against Intestinal Worms Affecting Millions
2025-01-13
Author: Siti
Introduction
A groundbreaking randomized controlled trial has revealed that a single, flavorful mango tablet combining two of the most widely used treatments for neglected tropical diseases holds significant promise for controlling intestinal worm infections, which affect an estimated 1.5 billion people each year. The study was highlighted in a recent publication in *The Lancet Infectious Diseases*.
Study Overview
Carried out on school-aged children in Ethiopia, Kenya, and Mozambique, the trial evaluated a fixed-dose combination (FDC) of ivermectin and albendazole. The results were striking; this new treatment proved to be more effective against *Trichuris trichiura* (whipworm) and other common soil-transmitted helminths (STH) than albendazole used alone, while maintaining a similar safety profile. Notably, the children found the treatment to be well-accepted, indicating a new level of pediatric compliance.
Impact of STH Infections
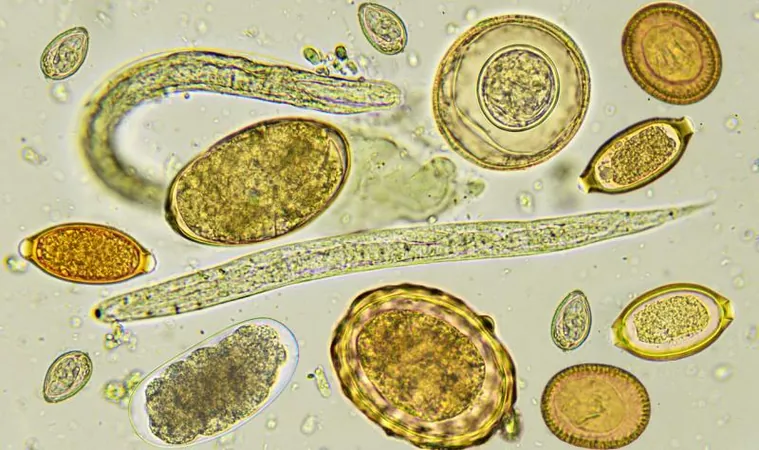
STH infections stem from the ingestion of eggs released by whipworms, roundworms (*Ascaris lumbricoides*), and hookworms (*Necator americanus* and *Ancylostoma duodenale*), often found in contaminated soil and water. These infections predominantly occur in tropical and subtropical regions characterized by inadequate sanitation. While many cases remain asymptomatic, they are capable of causing anemia and chronic protein deficiency, particularly affecting children and pregnant women—two of the most vulnerable populations.
Current Treatments and Challenges
The World Health Organization (WHO) has recommended preventive therapy using single doses of albendazole or mebendazole to at-risk populations for decades. However, there have been growing concerns about the efficacy of these treatments against *T. trichiura*, along with the risk of emerging resistance to these drugs. Furthermore, another intestinal parasite—*Strongyloides stercoralis*—does not respond to albendazole or mebendazole but has shown a strong susceptibility to ivermectin, making the FDC an even more critical breakthrough.
Trial Details
The purpose of this phase 2/3 trial, led by the STOP consortium (Stop Transmission of Intestinal Parasites), was to determine the safety and effectiveness of the novel orodispersible tablet combining ivermectin and albendazole. Participants included 1,001 children aged 5 to 18 from 15 schools across the three nations, with others presenting infections such as hookworm and *S. stercoralis*.
Key Findings
Key findings showed that both FDC treatment groups achieved significantly higher cure rates for *T. trichiura*: 97.2% for those receiving three doses over three days, and 82.9% for those receiving a single dose, compared to just 35.9% for albendazole alone. A notable increase in effectiveness was also observed in treating hookworm infections.
Potential for Treating Strongyloides Infections
While the sample size for *S. stercoralis* was too small to draw firm conclusions, preliminary results suggest that the FDC may also be effective against this stubborn intestinal parasite, given the existing efficacy of ivermectin.
Participant Feedback
Feedback from participants about the tablet’s palatability was overwhelmingly positive, with 93% liking the taste, 82% the smell, and 86% appreciating the texture. Such high acceptability rates bode well for future distribution.
Implications of the Findings
The implications of these findings are substantial, potentially revolutionizing current strategies to control STH infections and other tropical diseases. The WHO primarily recommends periodic de-worming for at-risk populations; however, the easy-to-administer nature of this new single-dose treatment paves the way for mass de-worming campaigns, while the three-day regimen could be tailored for individuals needing targeted therapy.
Expert Commentary
"This pivotal trial not only sets the stage for improved control of all STH species, including *Strongyloides*, but may also shift our goals for elimination that previously seemed out of reach with albendazole alone," commented Dr. Alejandro Krolewiecki, the clinical trial coordinator and first author from the Barcelona Institute for Global Health. He further noted that the next phase would involve extensive research in larger studies across other endemic regions and the practical feasibility of implementing this innovative treatment widely.
Conclusion
As the world grapples with the challenge of neglected tropical diseases, this breakthrough could represent a significant leap forward in our efforts to protect millions of vulnerable lives.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)