Revolutionary Gel for Bone and Tissue Regeneration Proves Successful in Rat Models!
2024-11-18
Author: Daniel
Groundbreaking Development in Biocooperative Materials
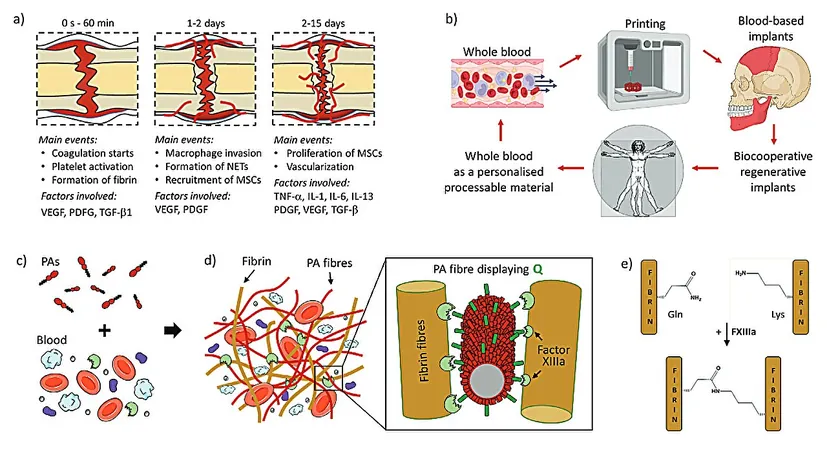
In a groundbreaking development, scientists from Queen Mary University of London and the University of Nottingham have unveiled a novel biocooperative material that fuses blood clotting mechanisms with peptide self-assembly. This innovative approach aims to engineer personalized regenerative implants, potentially revolutionizing the healing of severe wounds and fractures.
Advancements in Regenerative Therapies
As advancements in scientific knowledge and technology continue to surge, effective regenerative therapies seem closer than ever to becoming a reality in clinical settings. However, replicating the body's intricate healing environment presents a daunting challenge for researchers. Traditional methods often depend on stem cells, biomimetic materials, or allogeneic grafts—each posing unique hurdles in their pursuit of efficiency and reliability.
Understanding the Healing Process
The human body has evolved remarkably to heal minor injuries autonomously, primarily through a regenerative hematoma. This dynamic process orchestrates numerous molecular and cellular mechanisms that ensure full repair. Yet, significant strides are needed to enhance the healing capabilities for more severe injuries.
Research Study and Innovations
A recent study titled "Biocooperative Regenerative Materials by Harnessing Blood-Clotting and Peptide Self-Assembly," published in the journal Advanced Materials, showcases how researchers have developed peptide amphiphiles (PAs) that engage with blood components during coagulation. This interaction yields a living material that closely replicates the regenerative hematoma—an essential element in the healing process.
Innovative Methodology and Testing
The research team's innovative methodology co-assembled PAs with a patient’s own blood components to produce hydrogels that retained the structural and compositional attributes of the regenerative hematoma. The design incorporated varying charge densities in the PAs, allowing them to effectively interact with crucial proteins like fibrinogen and albumin. This also included glutamine residues, enabling the enzyme Factor XIIIa to cross-link PAs with fibrin, thereby enhancing the material's mechanical integrity.
Mechanical Properties and Microscopy Findings
Mechanical testing indicated that the gel could achieve a tunable stiffness and maintain a robust network architecture. Scanning electron microscopy provided visual evidence of a composite nanofibrous structure that mimics natural blood clots, demonstrating normal platelet adherence and spreading—crucial traits for effective healing.
In Vivo Experiment Results
Notably, this new regenerative gel not only shows compatibility with 3D printing techniques but also enables the fabrication of personalized implants on-site, tailored precisely to patient needs. In vivo experiments utilizing a rat skull defect model have yielded impressive results: the PA-blood gel implants stimulated bone regeneration with new bone formation rates of 62% and 56%, significantly outpacing the 50% achieved with the commercially available Bio-Oss and the mere 30% observed in control defects.
Future Directions in Regenerative Medicine
This research underscores a profound opportunity to leverage the intricate healing processes evolved over billions of years to develop cutting-edge regenerative therapies. While translating these findings to human medicine still faces challenges, this study illuminates a promising pathway toward more accessible and personalized regenerative healing solutions.
Conclusion and Future Updates
Stay tuned for more updates as this exciting field continues to evolve—could the future of regenerative medicine be just around the corner?



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)