Revolutionary Light-Guided siRNA Delivery System Developed from Cyanobacteria Promises Breakthroughs in Cancer Immunotherapy!
2024-11-26
Author: Siti
In a groundbreaking study published in Cell Reports Physical Science on November 25, researchers unveiled a game-changing light-guided biohybrid system named CTPA/siCSF1R, aimed specifically at targeting tumor-associated macrophages (TAMs). This innovative technology enables precise and temporal siRNA delivery, paving the way for enhanced tumor microenvironment modulation and more effective photoimmunotherapy.
Tumor-associated macrophages play a pivotal role in the immune response within tumors and are increasingly identified as promising targets for immunotherapy. However, existing gene therapy systems have struggled with significant limitations, including ineffective targeting, poor lysosomal escape, and the complications presented by a hypoxic, immunosuppressive tumor microenvironment. These challenges have compromised the clinical effectiveness of conventional gene carriers.
In a remarkable twist, this research team incorporated the cyanobacterium Synechocystis sp. PCC6803—often referred to simply as "cyan"—as a living biological carrier. This species showcases extraordinary self-driven tumor localization and phototactic abilities, making it an exceptional vehicle for precise drug delivery, particularly when augmented with controllable external light sources. By leveraging cyan's natural phototactic behavior and its ability to produce oxygen via photosynthesis, researchers believe this system stands to revolutionize nucleic acid drug delivery to solid tumors.
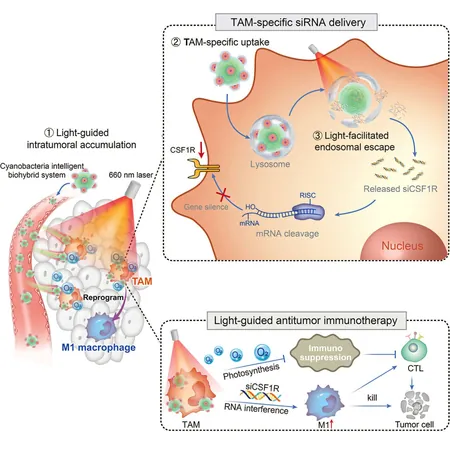
The structure of the CTPA/siCSF1R system comprises a triblock polyamino acid (TPA) gene vector designed to encapsulate siRNA, which is then conjugated to the surface of the photosynthetic cyanobacteria. This unique combination allows for the precise targeting of TAMs directly within the tumor's microenvironment, driven by the inherent properties of the cyanobacteria.
Moreover, the system utilizes the light-induced production of reactive oxygen species by the cyanobacteria, paired with the protonation of TPAs, to compromise the lysosomal membrane integrity. This disruption facilitates the release of siRNA directly into the cytoplasm of TAMs, enhancing therapeutic outcomes. The oxygen generated by the cyanobacteria during photosynthesis also plays a crucial role in improving the tumor microenvironment, boosting the effectiveness of the siRNA delivery and subsequent TAM reprogramming.
Encouraging results from experimental analyses demonstrated that the CTPA/siCSF1R system effectively reprograms TAMs towards the M1 phenotype—a state associated with heightened anti-tumoral activity. This reprogramming triggers an influx of pro-inflammatory cytokines, culminating in a robust immune response that significantly inhibits tumor growth. Remarkably, this innovative system exhibits excellent biosafety, showing no significant toxicity to the host organism, which is a critical consideration for potential clinical applications.
This revolutionary approach not only signals a new era in nucleic acid delivery vectors but also sets the stage for the advancement of tumor photoimmunotherapy. As the findings gain traction, scientists and medical professionals alike eagerly anticipate the implications for future cancer treatments.
Prof. Cai Lintao of the Shenzhen Institutes of Advanced Technology (SIAT), part of the Chinese Academy of Sciences, spearheaded this transformative research initiative, underscoring the remarkable potential of integrating biological innovation with therapeutic strategies. As the medical community looks ahead, one thing is clear: this pioneering system could very well reshape the landscape of cancer immunotherapy!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)