Revolutionary New Molecule Design Promises Safer Lithium-Ion Batteries
2024-10-03
Introduction
In an exciting breakthrough, researchers at Cornell University have developed a cutting-edge porous crystal that could be the foundation for safer lithium-ion batteries. By ingeniously fusing two unique molecular structures, they created a design that enables efficient lithium-ion transport through one-dimensional nanochannels, presenting a major leap towards solid-state battery technology.
Study Overview
The groundbreaking study, titled "Supramolecular Assembly of Fused Macrocycle-Cage Molecules for Fast Lithium-Ion Transport," has just been published in the prestigious *Journal of the American Chemical Society*. The research was spearheaded by Yu Zhong, an assistant professor in materials science and engineering, alongside lead author Yuzhe Wang, a passionate undergraduate student.
Background and Challenges
Zhong, who joined Cornell's faculty only two years ago, initiated this project with the goal of addressing the safety concerns associated with traditional lithium-ion batteries. Typical designs use liquid electrolytes, which can lead to dangerous situations like dendrite formation—sharp, spiky growths that can cause battery failures or even explosions. The team sought a solid-state alternative that mitigates these risks while still facilitating efficient ion movement.
Innovative Approach
A solid-state battery generally encounters the challenge of slower ion movement due to increased resistance in solid materials. To combat this, the researchers aimed to create a crystal structure that would offer porous pathways for smooth ion transport without hindering their movement. This required a delicate balance between holding sufficient lithium ions and maintaining low interaction forces to prevent ions from sticking to the crystal surfaces.
Contributions of Yuzhe Wang
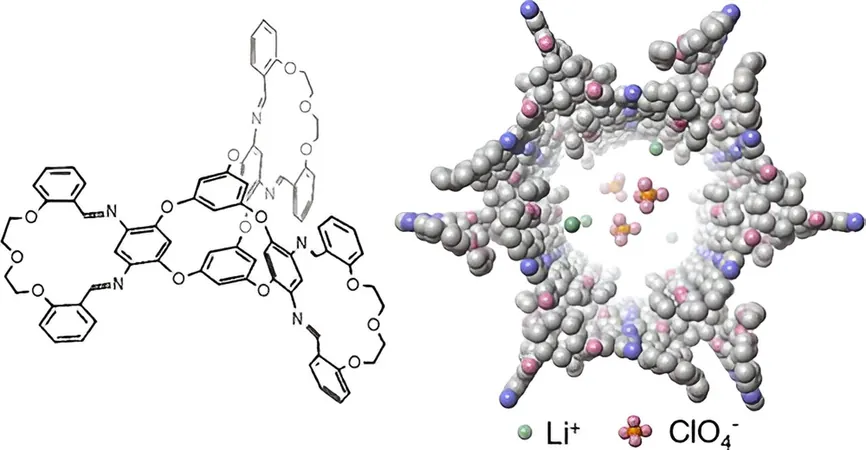
Wang's pivotal contribution involved synthesizing a unique fusion of macrocycles—large cyclic molecules comprising a ring of 12 or more atoms—and molecular cages, which resemble complex, multi-ring structures. Both components inherently contain pores capable of housing and transporting ions. By creatively combining these two elements, Wang fabricated a molecule that forms intricate three-dimensional crystals featuring well-defined nanochannels—ideal for lithium-ion transport.
Record-Breaking Conductivity
Remarkably, the conductivity achieved with this innovative crystal stands at a record high of 8.3 × 10^-4 siemens per centimeter for molecule-based solid-state electrolytes. This feat opens new doors not just for battery safety but also sets a new standard in the field.
Collaborative Analysis
Collaborations with experts from Cornell, such as Judy Cha and Jingjie Yeo, enabled the team to further analyze the crystal's structure and molecular interactions via advanced imaging and simulations. Their findings elucidated why this novel structure is especially advantageous for ion transport dynamics.
Broader Implications
The implications of this research extend beyond lithium-ion batteries. The newly developed material holds promise for additional applications like ion and molecule separation in water purification systems, and the design of mixed ion-electron conductors for advanced bioelectronics and sensors.
Future Directions
Zhong highlights the innovative aspect of their work: “While macrocycles and molecular cages have been explored individually, our research dives deeper into leveraging their unique geometries to produce new, complex structures that have not been fully tapped into before. We are eager to expand our investigations into various molecule syntheses that could yield even more nanoporous materials with diverse applications.”
Conclusion
With the rise in demand for efficient and safer energy storage technologies, the Cornell team's findings signal a pivotal moment in the field, potentially transforming how we power our devices and approach energy sustainability on a broader scale.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)