Revolutionary Nitrogen Editing Technique Set to Transform Drug Discovery!
2025-01-06
Author: Ming
Revolutionary Nitrogen Editing Technique Set to Transform Drug Discovery!
In the world of pharmaceuticals, the smallest atoms can wield tremendous power — particularly nitrogen. A recent groundbreaking discovery from chemists at the University of Oklahoma reveals an innovative strategy that leverages the incorporation of an additional nitrogen atom into molecular structures, maximizing its pharmacological potential.
Staggeringly, over 80% of small-molecule drugs approved by the US Food and Drug Administration from 2013 to 2023 feature nitrogen-containing rings. This revelation underscores the importance of nitrogen in enhancing drug efficacy. The researchers' pioneering skeletal editing reactions allow for the precise alteration of nitrogen's position within a molecule, unlocking new avenues for exploration in drug development.
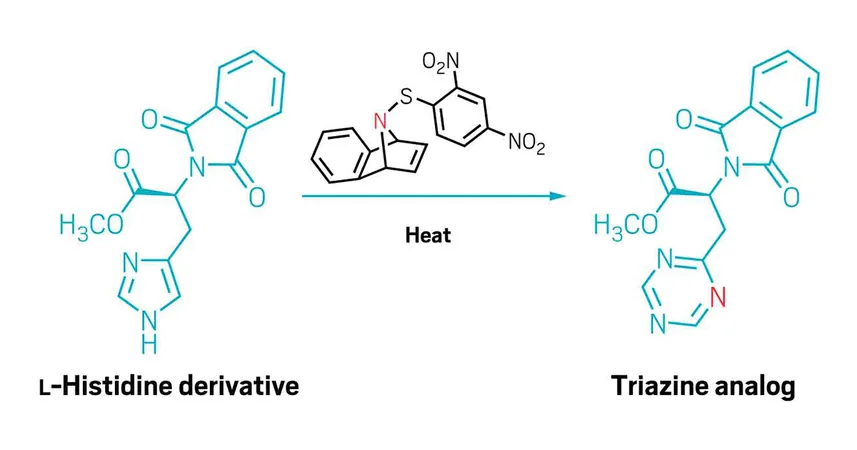
The team led by Indrajeet Sharma unveiled an advanced technique for extending five-membered aromatic rings already possessing a nitrogen atom by seamlessly adding a sixth nitrogen to the structure, an achievement detailed in their recent publication. Remarkably, this new reagent operates without additives and does not necessitate the protection of the existing nitrogen, making it invaluable for delicate modifications in late-stage drug development.
"This method offers adaptability across a variety of functional groups," states Sharma. The secret to their success lies in the use of sulfenylnitrenes—reactive compounds combining nitrogen and sulfur that act as both stabilizers and leaving groups during the reaction process.
Their research demonstrates versatility, as the sulfenylnitrene approach effectively introduces nitrogen into a range of five-membered aromatic N-heterocycles, including pyrroles, indoles, azaindoles, and imidazoles. Notably, these structures have historically posed challenges for nitrogen insertion reactions, making this advancement a game-changer for chemists.
The team illustrated their technique's broad functional-group compatibility through the modification of various complex molecules, including amino acid derivatives and oxidation-sensitive thioethers, positioning this method as a critical tool in pharmaceutical chemistry.
As they work to expand their technique to include rings devoid of nitrogen and to enhance reaction yields in safer solvents preferred by commercial labs, Sharma envisions a synergistic future where artificial intelligence collaborates with skeletal-editing chemistry. This innovative integration could enable rapid adjustments to drug candidates faltering in the development pipeline, showcasing the potential for swift “renovations” to existing drug structures rather than starting from scratch.
Richmond Sarpong from the University of California, Berkeley, applauded the team's approach, calling it "a wonderful addition to the growing armamentarium of single atom skeletal editing methods." As researchers eagerly anticipate the impact of this novel technique on drug discovery processes, the pharmaceutical industry may be standing at the brink of a revolutionary transformation in designing more effective therapeutics.
Stay tuned, as this exciting development could reshape the future of medicine!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)