Revolutionary Tool Unveils Protease Specificity: A Game Changer for Diabetes and Obesity Treatments!
2024-11-20
Author: Li
Groundbreaking Development at the Friedrich Miescher Institute
In a groundbreaking development, researchers at the Friedrich Miescher Institute (FMI) have unveiled an innovative tool that precisely maps how proteases—critical enzymes involved in protein processing—interact with their specific targets. This advancement not only challenges the long-standing perception of proteases as mere indiscriminate degraders but also opens up exciting avenues for targeted drug design aimed at combating diseases such as diabetes and obesity.
Introducing qPISA: A Novel Technique
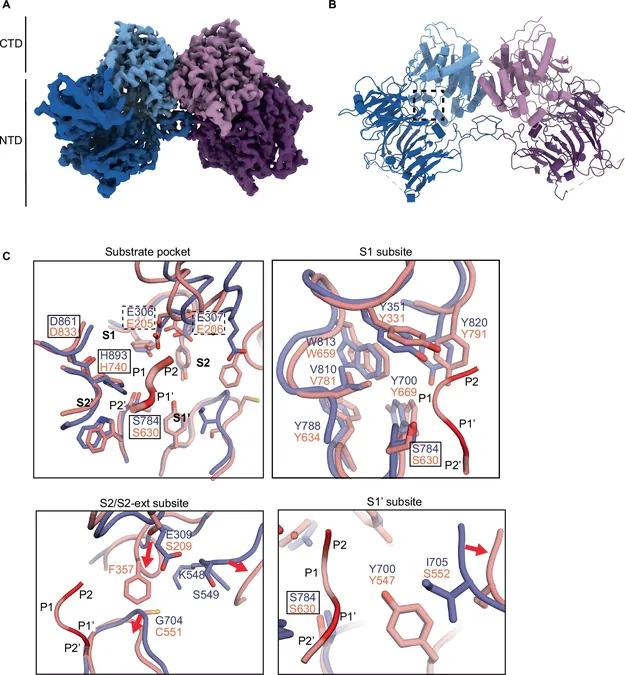
Typically focused on RNA and developmental timing, the Grosshans lab at FMI has pivoted towards creating a novel technique referred to as qPISA, which stands for "quantitative Protease specificity Inference from Substrate Analysis." This method enables scientists to delve deeper into the specificity of proteases, offering insights into their precise cleavage patterns—commonly known as "cleavage motifs"—that dictate which proteins they target.
The Specificity of Proteases
Lead author Helge Grosshans highlights, "While many believe proteases act randomly, our research demonstrates they possess a remarkable level of specificity." This is exemplified by the protease DPP4, which plays a crucial role in managing blood sugar levels by breaking down the peptide hormone GLP-1. Current medications that inhibit DPP4 or mimic GLP-1 are extensively utilized in treating type 2 diabetes and obesity, illustrating the enzyme's significant therapeutic potential.
Predicting Protein Stability with qPISA
Employing qPISA, the researchers were able to predict the stability or half-life of the protein targets of DPP4. With this insight, they explored modifications to GLP-1 with the goal of enhancing its stability and activity within the body—an essential step toward improving the efficacy of diabetes and obesity treatments, as emphasized by Grosshans.
Exploring DPF-3 and Its Unique Specificity Signature
In addition, the research team extended their study to a related enzyme known as DPF-3, found in the model organism Caenorhabditis elegans. By using advanced cryo-electron microscopy techniques, they mapped the structure of DPF-3, revealing significant structural similarities to human DPP4. However, the qPISA analysis uncovered a unique specificity signature for DPF-3, attributed to its intricate interactions with various substrates—further underscoring the complexities of protease behavior.
A Promising Future for Diabetes and Obesity Treatments
Grosshans concludes that this pioneering research contributes significantly to our understanding of protease selectivity and holds promise for developing more effective and longer-lasting treatments for type 2 diabetes and obesity. As we stand on the brink of a new era in drug development, this tool may very well transform current therapeutic strategies, paving the way for tailored medical interventions that could improve the quality of life for millions affected by these conditions.
Stay Tuned!
Stay tuned as we follow this exciting journey, where the marriage of cutting-edge research and therapeutic innovation could reshape the future of medicine!
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)