Revolutionary Ultrasound-Activated Drug Delivery System Set to Transform Medical Treatments
2024-12-27
Author: Daniel
Introduction
Researchers at the University of Utah are making waves in the medical field with a groundbreaking ultrasound-activated drug delivery system. Traditionally used primarily for medical imaging, ultrasound now shows promise in therapeutically delivering medications directly to specific areas of the body, potentially alleviating various disorders.
Targeted Drug Delivery
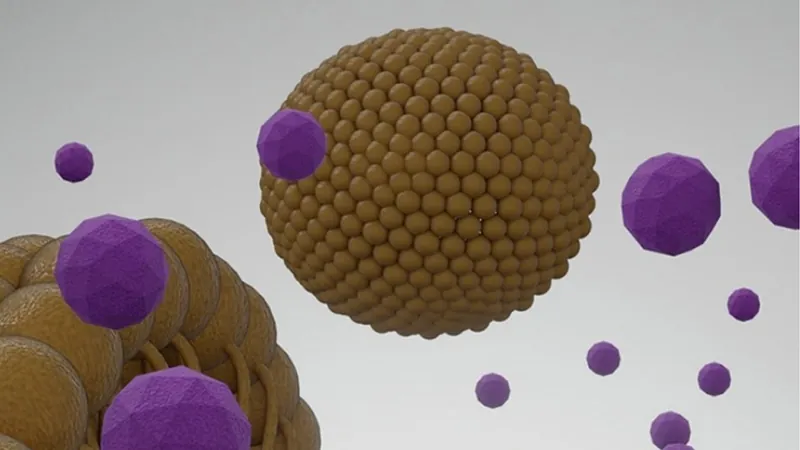
Imagine a future where medications are released only where needed at high concentrations—enhancing effectiveness while minimizing side effects. This has been a challenging task, particularly for conditions affecting delicate regions like the brain. However, researchers have crafted special nanoparticles that respond to focused ultrasound, releasing their drug payload exactly where needed.
Pioneering Study
In a pioneering study published in the Journal of Controlled Release, the team tested their innovative approach on non-human primates, successfully targeting the brain regions that require treatment. They effectively released the anesthetic propofol at precise locations, proving both safe and effective with reversible results.
Jan Kubanek, an assistant professor of biomedical engineering and lead author of the study, emphasized the primary advantage of this method. 'Using ultrasound-sensitive nanoparticles allows us to encapsulate the drug, significantly reducing unwanted interactions with the body until the ultrasound activates the release. This innovation could revolutionize the treatment of under-regulated or malfunctioning brain circuits without adverse effects on other brain regions.'
Design Innovations for Enhanced Stability
With support from the National Institutes of Health, the researchers engineered a cutting-edge, three-layer nanoparticle system. The inner core contains a contrast agent activated by ultrasound, while the second layer encapsulates the drug, and the outer shell protects against premature release.
Previous models faced stability issues in the bloodstream, posing safety risks. By refining their design—selecting a different contrast agent and reinforcing the outer shell—the team significantly increased the nanoparticles' stability. This improvement enables controlled drug release directly at the target site without affecting surrounding tissues until activated.
Promising Results in Large Animal Trials
In their experiments, the research team loaded the nanocarriers with a low dosage of propofol to assess safety and efficacy. They utilized a visual-choice paradigm—monkeys indicated which of two visual targets appeared first by moving their eyes. With a focus on the lateral geniculate nuclei (LGN), key brain areas for vision, they monitored how the drug's release influenced the monkeys' visual choices.
The results were striking. When propofol was administered to one side of the LGN, the corresponding visual perception on the opposite side was impaired. This indicated that the targeted drug release could expertly influence specific neural circuits.
The research found that mere 30-minute circulation of the nanoparticles in the bloodstream affords an efficient window for potential applications in human medicine. The method demonstrated a profound impact on the visual behavior of the primates only when the nanoparticles were activated, reaffirming the system's precision.
Guoying Liu, director at the National Institute of Biomedical Imaging and Bioengineering, stated, 'This study showcases near-real-time, safe drug release in awake primates—a significant advancement over earlier rodent studies, paving the way for clinical translation.'
Looking Ahead: A Future of Targeted Therapies
Aspiring to broaden the application of this technology, the research team sees potential across a variety of medications prone to side effects, including chemotherapy treatments. 'One of the brilliant aspects of our approach is that these nanoparticles can carry any drug and release it upon ultrasound activation,' Kubanek noted. 'We aim to use this system for treating cancer, managing pain, or breaking addiction cycles.'
Currently, they are undergoing further testing in non-human primates and exploring the delivery of chemotherapy via this innovative system in mouse models of glioblastoma, a notoriously aggressive and lethal brain cancer.
The future of drug delivery is bright, and this innovative method could redefine how we manage complex medical conditions. Stay tuned for more updates on this revolutionary research!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)