Revolutionizing the Chemical Industry: Researchers Unveil Groundbreaking Method to Slash Carbon Footprint!
2024-10-09
Author: Yu
A Breakthrough for Sustainability in Chemical Manufacturing
In a remarkable breakthrough for sustainability, researchers from Yokohama National University have unveiled a revolutionary method aimed at significantly reducing the carbon footprint of the chemical manufacturing industry—a sector notorious for its massive energy consumption and greenhouse gas emissions. This innovative research published in the Journal of the American Chemical Society on October 7 has the potential to reshape how chemicals are produced, turning the tide towards greener practices.
Cyclic Amines: The Heart of the Study
At the heart of this study are cyclic amines, essential building blocks for fine chemicals that play critical roles in products ranging from pharmaceuticals to everyday materials. Among these compounds, pyridine and its derivative piperidine are of utmost importance. Piperidine is utilized in a variety of applications, including FDA-approved drugs and pesticides, underscoring its significance to public health and safety.
The Limitations of Traditional Hydrogenation Processes
Traditionally, hydrogenation processes to add hydrogen to nitrogen-containing cyclic amines rely heavily on hydrogen derived from methane through a steam reforming process—an energy-intensive method responsible for approximately 3% of global carbon dioxide emissions. This reliance on fossil fuels not only exacerbates environmental problems but also represents a burden on energy resources.
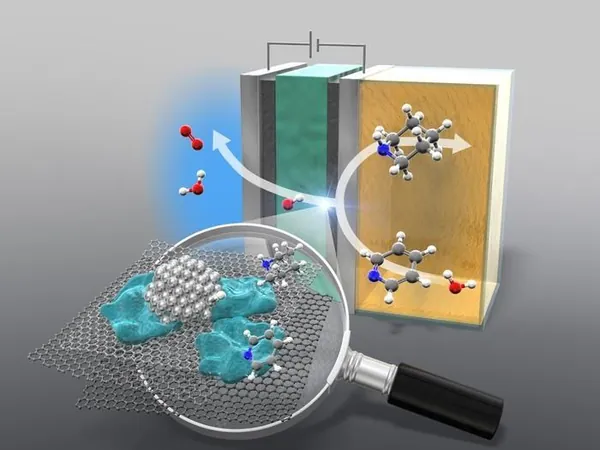
Innovative Anion-Exchange Membrane (AEM) Electrolyzer
However, the innovative solution put forward by the researchers is an anion-exchange membrane (AEM) electrolyzer, designed to enable hydrogenation at ambient temperature and pressure without the use of acidic additives that are typically required in conventional methods. Instead of relying on hydrogen gas, this electrolyzer splits water into atomic hydrogen and oxygen, allowing for a cleaner and more energy-efficient process.
Potential for Industrial-Scale Applications
"This method offers significant potential for industrial-scale applications in pharmaceuticals and fine chemicals, contributing to the reduction of carbon emissions and advancing sustainable chemistry," said Naoki Shida, the first author of the study. The ability of the AEM electrolyzer to operate under mild conditions means that the energy required for the process is notably less than traditional methods, enhancing its overall efficiency.
Impressive Yields and Future Prospects
Not only does this groundbreaking method use water and renewable electricity as energy sources, it boasts an impressive 78% yield when scaled up—making it both practical and scalable for industrial applications. While there may be challenges related to increased cell voltage during the electrolysis process, the researchers are optimistic that ongoing improvements in AEM technology will address these issues, paving the way for widespread adoption.
A Paradigm Shift Towards Sustainable Chemistry
The implications of this technology are staggering; if implemented on a broader scale, it could initiate a paradigm shift within the chemical industry, marking a move away from fossil fuels towards a more sustainable future. As companies prioritize greener practices in response to climate change, this advancement in sustainable chemistry could be the game-changer that enables the industry to significantly lower its carbon footprint.
Conclusion: A Vital Step Towards Sustainability
As the world grapples with the pressing challenges of climate change, the findings from this research represent not just a scientific breakthrough, but a vital step toward a more sustainable and environmentally responsible chemical industry. Will this method be the key to transforming manufacturing practices and reducing emissions on a global scale? Time will tell, but the momentum is certainly building!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)