Shocking Discovery! Copper's Role in Photocatalysts is Not What We Thought!
2024-11-25
Author: Li
New Insights into Copper's Behavior in Solar Fuel Production
Copper has long been hailed as a potential superstar in the quest for sustainable fuel conversion, particularly in the transformation of carbon dioxide into energy-rich compounds. This process is crucial for not only reducing greenhouse gases but also for developing renewable energy sources. While many researchers believed that copper would help produce more electron-rich species under solar energy, recent discoveries have flipped this idea on its head.
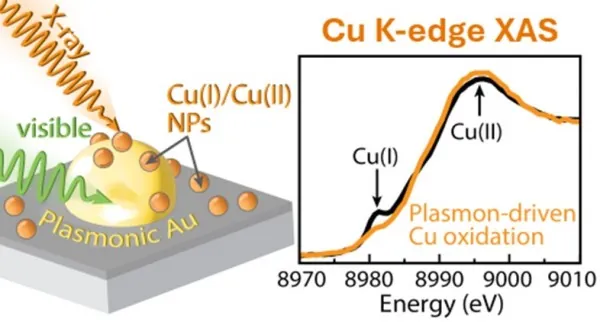
A groundbreaking study employing advanced X-ray techniques unveiled that copper behaves quite unpredictably when utilized solely with light—without any electrical assistance. The research unveiled that instead of yielding reduced chemicals, copper forms more oxidized species during photocatalytic reactions. This unexpected revelation is paving the way for a deeper understanding of photocatalytic reactions and highlights copper's latent complexities.
Why This Matters
The ability to harness copper effectively for carbon capture can significantly impact future energy technologies, especially in creating solar fuels. This study's findings suggest that when copper is combined with a common plasmonic material (like gold), it behaves quite differently from traditional expectations. In plasmonic materials, electrons are able to vibrate in sync, and this interaction was found to trigger a hole transfer from gold to copper when exposed to light. Such a phenomenon indicates that the reaction dynamics must be reconsidered for effective catalytic design.
Key Research Findings
The research team, part of the Liquid Sunlight Alliance (LiSA) at the Stanford Synchrotron Radiation Lightsource, conducted detailed investigations using Cu K-edge X-ray absorption spectroscopy. Their trials explored the behavior of copper in conditions mimicking gas-phase carbon dioxide absorption with water vapor. They astonishingly identified that under visible light irradiation, copper becomes increasingly oxidized, transforming from metallic copper to a mixture of Cu(I) and Cu(II) oxides, hydroxides, and carbonates.
These insights effectively demonstrate that, in light-driven environments, copper takes on an oxidative role in photocatalysis instead of a reductive one—a major paradigm shift that could influence how future photocatalytic processes are designed and optimized.
Looking Ahead
The implications of these findings are vast. By delving into how copper interacts with light and other materials, researchers are unlocking new pathways for efficient carbon dioxide conversion. With continuous support from the Department of Energy and further exploration, this research not only boosts our understanding of photocatalysis but may also catalyze breakthroughs in solar energy applications and carbon capture technologies.
Stay tuned for more updates as this exciting field of research progresses!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)