Shocking Estimate Reveals More Than Half of U.S. Adults Qualify for GLP-1 Medications!
2024-11-23
Author: Sarah
Groundbreaking Analysis by BIDMC Researchers
In a groundbreaking analysis by researchers at the Richard A. and Susan F. Smith Center for Outcomes Research at Beth Israel Deaconess Medical Center (BIDMC), it has been discovered that an astonishing 137 million U.S. adults—over half of the adult population—are eligible for semaglutide, a medication primarily used for weight loss, diabetes management, and the prevention of recurrent cardiovascular events.
Urgent Need for Equitable Access
Presented at the prestigious American Heart Association Scientific Sessions and featured in JAMA Cardiology, the study highlights the urgent need for equitable access to this new class of life-changing pharmaceuticals. "These staggering numbers indicate a future surge in spending on semaglutide and related medications," warned Dr. Dhruv S. Kazi, the study’s lead author and associate director of the Smith Center. "It is critical for both clinicians and policymakers to prioritize equitable access to these effective yet expensive treatments and offer support to individuals to ensure they can maintain long-term therapy."
The Benefits of Semaglutide
Semaglutide is classified as a GLP-1 receptor agonist and is currently approved for managing diabetes, treating obesity, and preventing recurrent cardiovascular disease, particularly among individuals with a history of heart attacks or strokes, or who suffer from peripheral artery disease. Despite the vast pool of eligible adults, currently, only about 15 million—just over 10%—are using semaglutide. Notably, it emerged as the top-selling drug in the U.S. in 2023, based on overall pharmaceutical spending.
Emerging Research on Semaglutide
Emerging research continues to shed light on semaglutide’s potential benefits beyond its initial approvals. Studies show that this medication not only aids in managing diabetes and weight loss but may also alleviate symptoms of sleep apnea, improve conditions related to certain types of heart failure, and slow down the progression of chronic kidney disease. Furthermore, semaglutide and other drugs belonging to its class are under investigation for their effectiveness in treating liver and kidney diseases, substance use disorders, and even dementia.
Analysis Methodology
The analysis conducted by Dr. Ivy Shi, an internal medicine resident at BIDMC, utilized five years of data from the National Health and Nutrition Examination Survey—an extensive health survey managed by the U.S. Department of Health and Human Services. The researchers assessed health data from 25,531 U.S. adults aged 18 and older to determine eligibility for semaglutide based on established indications.
Striking Results
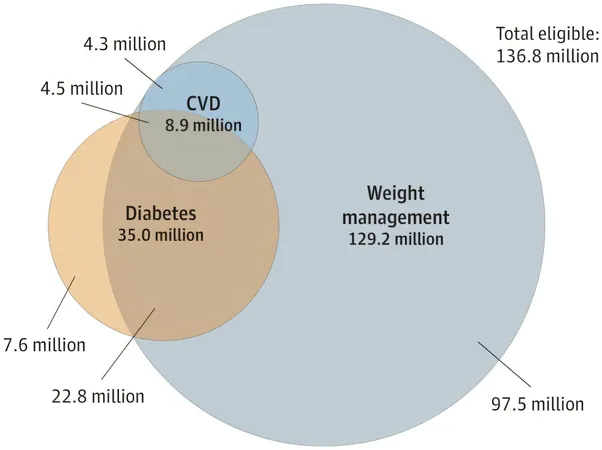
The results were striking: Among the 136.8 million Americans eligible for semaglutide, approximately 35 million qualify for diabetes management, while a staggering 129.2 million are eligible for weight loss treatment. Moreover, 8.9 million adults can benefit from semaglutide for the secondary prevention of cardiovascular disease. The scope of potential beneficiaries spans various insurance coverage, including 26.8 million relying on Medicare, 13.8 million on Medicaid, and 61.1 million with commercial insurance plans.
Conclusion: A Shift in Health Management
As the impact of semaglutide continues to unfold, it underscores an essential shift in how we approach health management and wellness strategies, promising a healthier future for millions. Stay tuned, as the full implications of this study could revolutionize how we view treatments for weight loss and chronic diseases!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)