Shocking Revelations: Copper Takes a Surprising Turn in Carbon Dioxide Conversion!
2024-11-26
Author: Wei Ling
Copper's Role in Carbon Dioxide Conversion
Copper has emerged as an intriguing player in the quest for sustainable energy solutions, particularly in transforming carbon dioxide into more reactive substances known as reduced species. This pivotal reaction is essential in the development of solar fuels, and while we often rely on electrical energy to kickstart this process, recent breakthroughs suggest that harnessing solar energy could be even more effective.
The Enigma of Copper Catalysts
But what if I told you that our understanding of how copper acts as a catalyst during these solar reactions is still murky? Researchers are delving into this enigma, employing advanced X-ray techniques to uncover how copper catalysts behave when subjected solely to light—without any electrical input.
Revolutionary Findings
Their findings are nothing short of revolutionary: rather than producing reduced species, as previously believed, copper instead generates more oxidized chemical compounds during these reactions. This surprising behavior underscores the importance of examining how copper catalysts function when illuminated by sunlight, as it is crucial for utilizing them in carbon capture and conversion technologies.
Publication Insights from ACS Nano
A recent publication in ACS Nano highlights new insights into this phenomenon, revealing that when copper is paired with a widely-used plasmonic light-absorbing material, it forms oxidized species more readily. Plasmonics refers to the collective oscillations of electrons in metals, which dramatically enhance light absorption—a game-changer for catalytic efficiency.
Innovative Experimental Setup
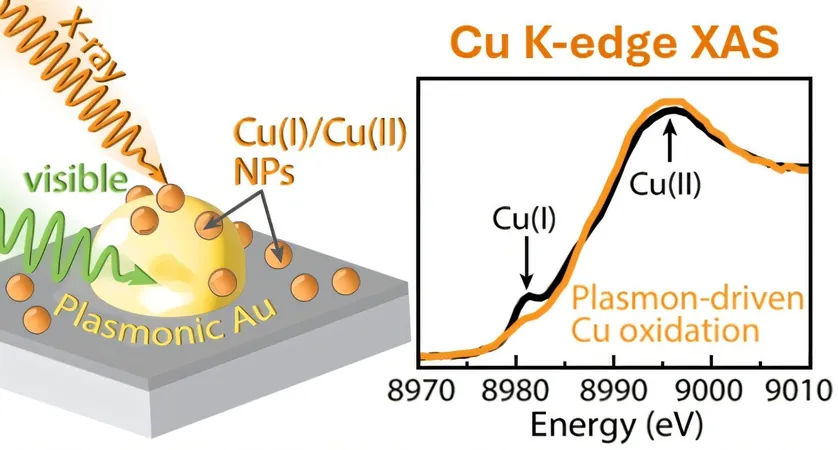
The researchers demonstrated that photocatalytic conversion of carbon dioxide to carbon monoxide could occur without any electrical assistance by employing a unique combination of materials: a p-type gallium-nitride (GaN) semiconductor, plasmonic gold light absorbers, and copper co-catalysts. This innovative setup was scrutinized by scientists at the Liquid Sunlight Alliance (LiSA) Fuels from Sunlight Energy Innovation Hub, revealing new insights into copper's role in this effective system.
Significant Transformations Observed
Utilizing Cu K-edge X-ray absorption spectroscopy at the Stanford Synchrotron Radiation Lightsource, the researchers found that when exposed to carbon dioxide and water vapor, the copper underwent a significant transformation. They identified a mixture of Cu(I) and Cu(II) oxide, hydroxide, and carbonate compounds, revealing that no metallic copper was present during the reaction.
Light-Driven Electron Transfer
Under visible light conditions, further oxidation of the copper was observed, suggesting that light facilitated an electron transfer process from gold (Au) to copper (Cu). This was corroborated by computational modeling, which indicated that such light-driven hole transfer was thermodynamically favorable.
Implications for Photocatalysis
What does this mean for the future of photocatalysis? It opens up a frontier of possibilities, showcasing that copper may take on a more oxidative role rather than a reductive one when paired with plasmonic materials for light absorption. As researchers continue to explore the vast potentials of copper catalysts, the implications for solar-driven carbon conversion technologies could be transformative—and may just revolutionize how we think about sustainable energy. Stay tuned, as we unravel the mysteries of photocatalysts and their untapped potential!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)