The Race for Alzheimer’s Blood Tests: Are We on the Verge of a Breakthrough?
2024-11-21
Author: Nur
The Race for Alzheimer’s Blood Tests: Are We on the Verge of a Breakthrough?
On November 21, 2024, at the CTAD conference held in Madrid, experts voiced their excitement regarding the rapid advancement of blood tests designed for the early diagnosis of Alzheimer’s disease. Suzanne Schindler from Washington University, St. Louis, made headlines with her statement that the field is at a transformative moment, emphasizing that cutting-edge plasma tests are set to revolutionize Alzheimer’s diagnostics.
"Things are moving very quickly," she stated, forecasting that we might see FDA-approved blood tests that also receive reimbursement in 2025. "This would significantly alter the current landscape of Alzheimer’s diagnostics."
Currently, the implementation of Alzheimer's testing in the UK is strikingly low, with only about 2 percent of eligible individuals undergoing the recommended CSF marker testing or PET scans. Jonathan Schott from the Queen Square Institute of Neurology believes that blood tests could push that number towards 100 percent. "We now have the chance to democratize the molecular diagnosis of Alzheimer’s," Schott proclaimed.
Several laboratory-developed tests (LDTs), particularly evaluating blood levels of p-tau217, are already available in the U.S. and Europe, making inroads in both research and specialized clinical practices. Companies like ALZpath Inc., C2N, Eli Lilly, LabCorp, and the Mayo Clinic are at the forefront. However, the FDA has set a deadline that by 2028 any new plasma diagnostic test for Alzheimer’s will require formal approval, prompting many companies to seek compliance to maintain a competitive edge.
Fujirebio recently filed for FDA approval for its plasma p-tau217/Aβ1-42 test and plans to pursue certification from European and Japanese authorities. Similarly, Roche Diagnostics confirmed their filings for IVD approval for p-tau181 and p-tau217 assays. Both companies received breakthrough designations from the FDA for their respective tests, showing the regulatory body’s recognition of the urgency behind Alzheimer’s diagnostics.
Industry players like Beckman Coulter Diagnostics are also on the move, with plans for future IVD certification submissions. The race is not just limited to established firms; startups like ALZpath are entering the fray by licensing proprietary antibodies for new diagnostic pathways. Experts expect more vendors to follow suit, including C2N Diagnostics and Eli Lilly, further propelling the speed of innovation in this critical area.
The swift progress is attributed to new diagnostic criteria established by a Global CEO Initiative panel. The recommendations stress the importance of achieving a sensitivity of at least 90% and specificity of 85% for blood biomarker tests, setting high standards that are driving vendors to refine their offerings.
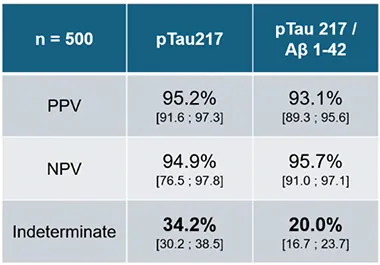
In recent studies conducted in Spain, researchers found promising results with the p-tau217/Aβ1-42 ratio outperforming p-tau217 alone. The robust predictive values, reaching 95% PPV and 96% NPV, have fueled optimism among scientists and stakeholders. These findings validate the potential of plasma tests to accurately identify Alzheimer’s disease, even in early or ambiguous cases.
As the scientific community anticipates the approval of these diagnostic tools, there is also an eagerness to understand how they will be integrated into clinical practice. Research is underway globally to assess the practical impacts of these blood tests, with forward-thinking studies examining their effects on diagnosis and treatment management.
However, experts warn against treating positive test results as definitive diagnoses. There is a pressing need for educational efforts targeting healthcare professionals to ensure these tests are used appropriately. Guidelines for the proper use of these diagnostics are in development, led by organizations like the Alzheimer’s Association, aiming to solidify clinical practices.
Despite the excitement surrounding these advancements, a cloud of uncertainty looms regarding insurance reimbursement rates for these tests. The proposed rate from the Centers for Medicare and Medicaid Services stands at $17.27, significantly lower than existing reimbursements for CSF tests, which are set at $130. There is a collective push from the scientific community for a reassessment of this reimbursement rate to ensure that labs can sustain the cost of implementing these advanced tests.
The landscape of Alzheimer’s diagnostics is rapidly evolving. With breakthroughs on the horizon, stakeholders are hopeful that soon managing and diagnosing Alzheimer's will become more accessible than ever before—potentially paving the way for improved patient outcomes across diverse populations.


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)