The Surprising Role of Netrin1 Protein in Spinal Cord Development That Could Change Everything!
2024-11-18
Author: Mei
Introduction
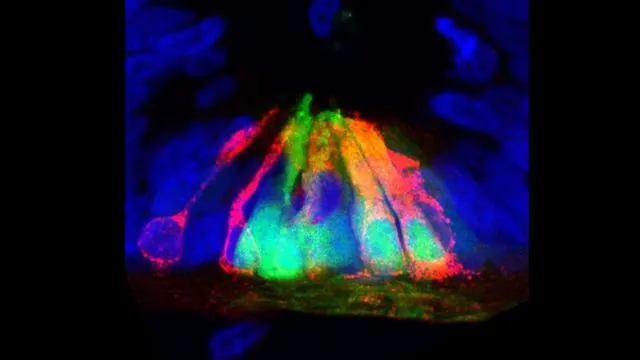
A groundbreaking discovery by scientists at UCLA's Eli and Edythe Broad Center of Regenerative Medicine and Stem Cell Research has unveiled an unexpected role for the molecule netrin1 in spinal cord development. While previously identified primarily as a guidance cue for nerve fibers, new research indicates that netrin1 plays a crucial role in maintaining the spatial limits of bone morphogenetic protein (BMP) signaling within the developing spinal cord.
The Role of Netrin1 in BMP Signaling
The researchers found that netrin1's boundary-setting capabilities are essential for normal sensory neuron development, as they direct BMP signaling specifically to the dorsal region of the spinal cord. This controlled signaling is paramount for the compartmentalization necessary for the proper formation of sensory pathways, such as those responsible for processing touch and pain.
Implications for Spinal Cord Repair
The exciting study, published in the Cell Reports, reshapes our understanding of spinal cord circuitry and suggests potential new strategies for spinal cord repair. Lead researcher Professor Samantha Butler, who heads the study, emphasized the thrill of scientific discovery by exploring unexpected findings. "Netrin1 has always been viewed as a critical architect of neural circuits, but its unexpected influence in organizing spinal cord architecture is groundbreaking,” she stated.
Distinction Between Neuronal Types
The role of netrin1 becomes even more significant in ensuring distinct boundaries between sensory neurons in the dorsal region and motor neurons in the ventral region. Disruptions in BMP signaling could result in a misaligned neural network, leading to sensory processing problems.
Challenging Traditional Theories
In a remarkable twist of research, Butler and her team previously modified the conventional understanding of how axons—nerve fibers connecting neural cells—navigate during embryonic development. Original theories posited that axons were attracted or repelled by guidance cues over long distances. However, Butler’s earlier research showed that netrin1 acts more like a sticky adhesive that guides axon growth directly along specific pathways.
Experimental Findings
Intrigued by these revelations, Butler’s team conducted gain-of-function experiments on chicken and mouse embryos, introducing a traceable version of netrin1. Surprisingly, they noted a disappearance of axons. Initially puzzled, graduate student Sandy Alvarez linked the phenomenon to netrin1’s ability to downregulate BMP signaling, showcasing how netrin1 represses BMP activity through control of RNA translation.
Therapeutic Implications
This nuanced interaction presents ground-breaking implications not just for spinal cord development but potentially for therapeutics targeting nerve damage. Butler expressed her ambition to harness netrin1's properties to rebuild neural circuits in patients suffering from spinal injuries, heralding a new frontier in regenerative medicine.
Broader Biological Impact
With the implications of this research extending to other organs where BMP and netrin1 play roles, such as in certain cancers or developmental disorders, the team urges a reassessment of our understanding of these molecules across biological systems. "Our findings may signal a paradigm shift in how we view the interactions of critical signaling molecules," Alvarez remarked.
Conclusion
As research on netrin1 continues, the potential for new treatments emerges, raising hope for individuals affected by spinal cord injuries or neural disorders. This exploration into netrin1 may hold the key to unlocking new avenues of intervention that could revolutionize neural repair methodologies. Stay tuned for future revelations from this groundbreaking research!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)