Unlocking the Mysteries of Autonomous Cellular Behavior During Early Development
2024-11-18
Author: Wei
Introduction
Have you ever wondered how a simple ball of cells transforms into a complex being? During the first weeks after fertilization, human embryonic cells engage in numerous divisions, creating a larger mass. However, everything changes during a critical stage called gastrulation—this is where our body plan is established. This phase transforms uniform cells into a structured, multi-layered organism complete with specialized cell types.
The Importance of Gastrulation
Gastrulation is not just any ordinary developmental step; it is the moment when our three body axes—head-to-tail, front-to-back, and left-to-right—are defined. Interestingly, this essential process involves intricate interactions between cells that coordinate with extraordinary precision. Yet, the underlying mechanisms of how gastrulation occurs remain largely unexplained.
Research Contributions from the Trivedi Group
Researchers from the Trivedi Group at EMBL Barcelona are making strides in demystifying this phase of development. According to Vikas Trivedi, the Group Leader, prior beliefs suggested that our body’s anterior-to-posterior axis—our head-to-feet arrangement—relied heavily on external signals for development. However, their recent findings published in the journal Development indicate a groundbreaking discovery: cells can independently initiate the early stages of symmetry breaking without outside assistance.
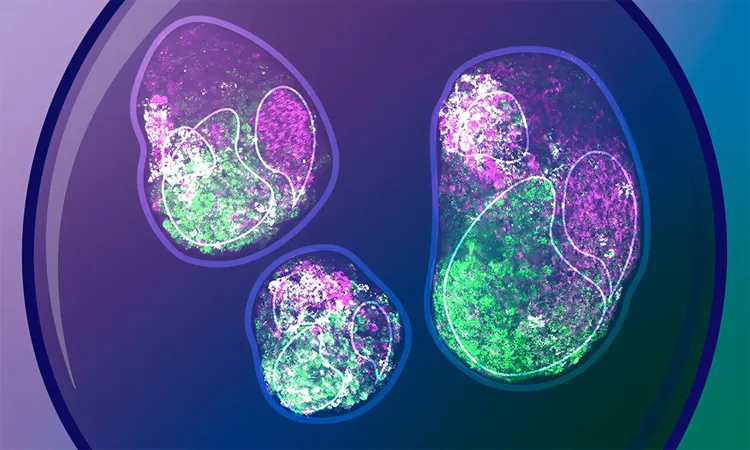
By using gastruloids—three-dimensional clusters of embryonic stem cells cultivated in laboratory conditions—scientists gain insights into early mammalian embryo structures, something that's challenging to observe directly in a living organism. What's astonishing is that these aggregates can form organized structures devoid of the typical patterns or signals that guide embryonic cells in the uterus. This opens the door to comprehending just how much potential embryonic cells possess to self-organize and form a comprehensive body plan.
Methodology and Findings
Kerim Anlas, a co-first author and former Ph.D. candidate in the Trivedi Group, emphasized the aim of their research: 'By studying gastruloids under minimal conditions, we hope to unveil key details about early embryonic development that are often overlooked when only examining native embryos.'
The Trivedi team's exploration involved growing gastruloids from mouse embryonic stem cells for the crucial initial 72 hours post-fertilization—the time when pivotal decisions regarding the head-to-tail axis are made. Throughout this period, they analyzed gene expression and other vital molecular markers in individual cells of the gastruloids.
Their observations revealed that these gastruloids could independently achieve symmetry breaking, with cells at both ends of the structure displaying distinct characteristics without needing external chemical stimulation. Remarkably, certain gastruloid cells began to express a gene known as Brachyury and underwent critical shape changes and increased cell motility. These processes eventually lead to the formation of the mesoderm, the germ layer that gives rise to vital structures such as bones and muscles.
Implications of Findings
This research marks the nascent stages of anterior-to-posterior axis formation, demonstrating that such developments can occur without external signals. Moreover, the team conducted comparative analysis between gastruloids and embryonic cells, revealing that despite the differences in the initial gene expression stages, both result in remarkably similar differentiated cell types.
Trivedi commented on this resemblance: 'This indicates that cells can reach the same end points in both gastruloids and living embryos, albeit starting from different places.'
Nicola Gritti, an image analysis specialist and former postdoctoral researcher in the Trivedi Group, voiced optimism regarding the potential of these findings. He noted that this research provides a fresh approach to studying early mammalian development. 'While many studies aim to recreate a perfect model of embryo development, our findings shed light on critical differences in embryonic events that demand further exploration,' he explained.
Conclusion and Future Directions
The implications of this research extend far beyond curiosity; they may redefine our understanding of developmental biology and offer new avenues for medical advancements. As these scientists continue to unlock the secrets of cellular autonomy during critical growth stages, we are left to ponder: what does the future hold for regenerative medicine and our ability to harness these processes? Stay tuned as this fascinating field evolves!

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)