Unlocking the Secret to Perfectly Folded Designer Proteins
2025-04-10
Author: John Tan
The Building Blocks of Life: Proteins
Proteins are the essence of life. These complex molecules are formed by folded chains of amino acids and serve numerous vital functions—from maintaining cellular structures to facilitating biochemical reactions. Their functionality is enhanced through various modifications after their initial synthesis.
The Mystery of Protein Splicing Unveiled
One fascinating modification process is protein splicing, where a segment known as 'intein' removes itself from the peptide chain. This self-removal is crucial to ensure that proteins fold correctly and operate efficiently.
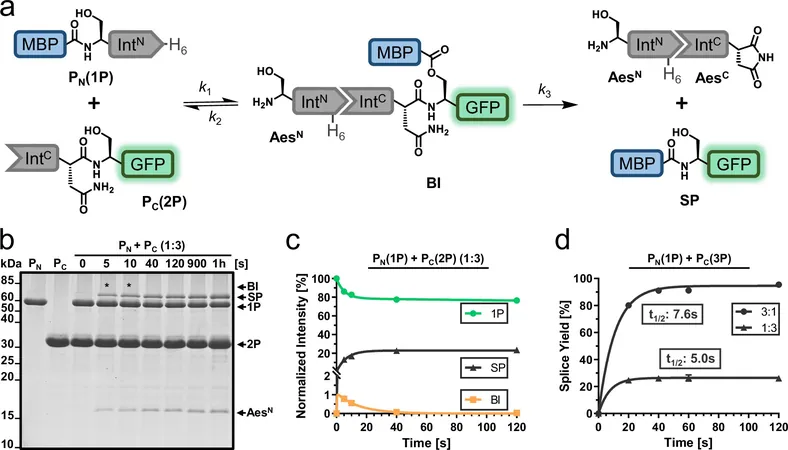
Breakthrough Research from Münster
A groundbreaking team, led by protein chemist Prof. Henning Mootz and Ph.D. student Christoph Humberg at the University of Münster, has cracked a long-standing dilemma: Why do 'split inteins,' a special type of intein, often struggle in lab settings, resulting in reduced reaction efficiency? The duo identified protein misfolding as a primary issue and devised an innovative method to combat it.
Implications for Biotechnology and Biomedicine
Published in the prestigious journal Nature Communications, their findings offer exciting prospects for utilizing split inteins to engineer proteins tailored for basic research and advanced applications in biotechnology and biomedicine.
The Quest for Complex Proteins
Global scientists are dedicated to synthesizing intricate proteins from two challenging fragments, often impossible to create via traditional methods. This innovative approach enables the crafting of chimeric proteins, where one segment could be derived from mammalian cells while the other is chemically synthesized or sourced from bacteria.
Harnessing Power of Split Inteins
For this sophisticated protein assembly, robust split inteins are essential. These remarkable tools merge separate protein segments by linking their initially distinct peptide chains before self-removing.
Investigation of the Aes Inteins
Focusing on the 'Aes intein'—known for its versatility in catalysis—the Münster team uncovered that low productivity rates mirrored findings from other inteins. Utilizing chromatographic and biophysical methods, they revealed that a significant portion of one fragment existed as an inactive protein aggregate due to specific misfolding.
Transforming Challenges into Solutions
From these crucial observations, the team pinpointed the amino acids responsible for the misfolding issues. By employing molecular biological techniques, they introduced targeted mutations to the intein fragment, successfully minimizing aggregate formation and significantly boosting productivity.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)