Unmasking the Power of AAV Vectors: Key Insights for Lung Gene Therapy
2024-11-25
Author: Yu
Unmasking the Power of AAV Vectors: Key Insights for Lung Gene Therapy
The future of genetic treatments for lung diseases like cystic fibrosis (CF) and chronic obstructive pulmonary disease (COPD) is looking bright, thanks to advancements in adeno-associated virus (AAV) vectors. In recent studies, researchers have highlighted the potential of AAV vectors as powerful tools for gene therapy because of their remarkable ability to target the airway epithelium effectively.
AAVs are small, non-pathogenic viruses with a diameter of only 20-25nm, categorized under the Dependoparvovirus genus of the Parvoviridae family. Found in a range of vertebrates, including humans, these vectors have garnered significant interest for their non-infectious nature, low immune response, and ability to provide prolonged gene expression without adverse side effects. The potential applications extend to treating a variety of pulmonary conditions, from hereditary ailments to infections like COVID-19.
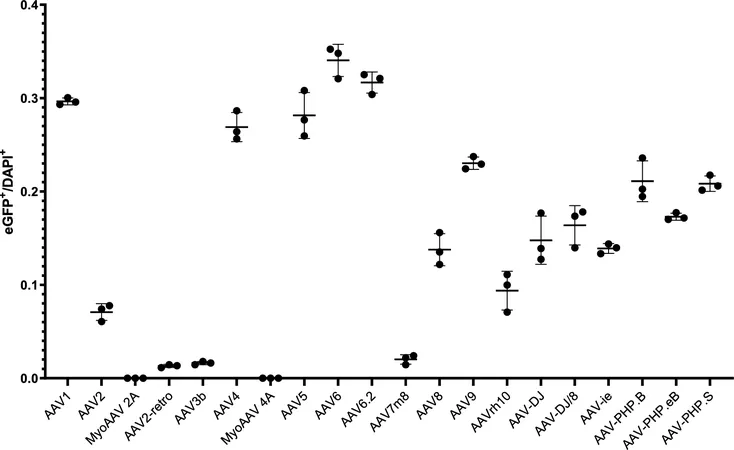
Research has shown that various AAV serotypes exhibit different efficiencies in infecting lung cells. A comprehensive study tested twenty commercially available AAV serotypes in mouse models to determine their tropism—essentially their targeting abilities within the lungs. The standout performers were AAV1, AAV4, AAV5, AAV6, AAV6.2, AAV9, and AAV-PHP.S, which displayed notably high transduction efficiency. Conversely, serotypes like AAV2 and AAV3b proved ineffective, suggesting that the choice of AAV could significantly influence the success of gene therapy initiatives in pulmonary applications.
Delving deeper, researchers employed advanced immunofluorescence techniques to discern which specific lung cell types were being targeted. The results illuminated that AAV1, AAV4, AAV5, AAV6, and AAV6.2 effectively transduce club cells, vital for maintaining airway health. Notably, AAV vectors also showed promise in infecting ciliated cells and alveolar type II cells, pinpointing their suitability for gene therapy strategies addressing conditions such as primary ciliary dyskinesia and pulmonary fibrosis.
While the findings are impressive, they also raise significant questions. Notably, the study indicated that current AAV serotypes seem ill-equipped for targeting neuroendocrine and airway smooth muscle cells, hinting at a gap in therapeutic potential for certain lung conditions. There is an urgent need to develop newer AAV serotypes that can effectively deliver genes to these specific cell types to advance gene therapy comprehensively.
It's essential to note that while these results are promising in mouse models, variations might exist in human lungs due to species-specific differences in lung biology. Therefore, supplementary research using human lung organoids and animal models like pigs and ferrets—similar in airway structure to humans—could aid in fine-tuning gene delivery strategies.
The method of AAV delivery through intratracheal instillation offers several advantages over systemic injection. This approach allows for localized treatment, minimizes potential systemic side effects, and improves the overall efficiency of gene delivery to lung cells. Such targeted methodologies are imperative for enhancing the outcomes of gene therapies for pulmonary diseases.
This systematic analysis of AAV vectors holds immense potential for revolutionizing lung gene therapy and underscores the importance of tailoring therapeutic approaches based on the specific cell types involved. As research continues, the possibility of tailored AAV vector designs adapted for various pulmonary ailments may soon transform the landscape of respiratory disease treatment.
Stay tuned for more breakthroughs in the battle against lung diseases—who knows what the future holds!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)