Unraveling the Complex Role of Fibroblast Subtypes in Skin Cancer: A Path Toward Revolutionary Immunotherapies
2024-11-19
Author: Siti
Recent research conducted at MedUni Vienna's Department of Dermatology sheds light on the diverse roles of cancer-associated fibroblasts (CAFs) in skin cancer, potentially paving the way for groundbreaking immunotherapy treatments. This revolutionary study, published in the esteemed journal *Nature Communications*, emphasizes the intricate relationship between CAFs and tumor progression in various skin cancer types, namely basal cell carcinoma, squamous cell carcinoma, and melanoma.
Fibroblasts, the body's connective tissue cells, are fundamental in wound healing and tissue repair. They are responsible for producing the extracellular matrix, which provides structural integrity to tissues by generating proteins like collagen. However, when it comes to cancer, fibroblasts can take on a more sinister role, becoming cancer-associated fibroblasts that contribute to tumor growth and therapeutic resistance.
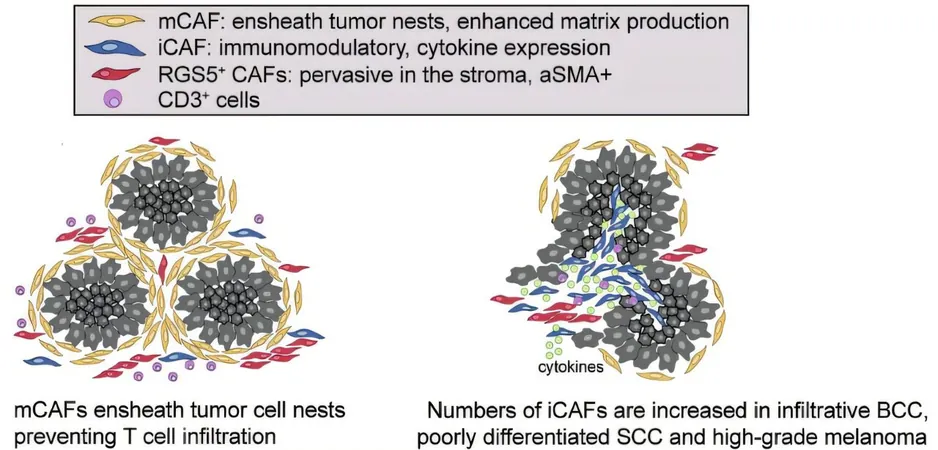
In a pioneering approach, MedUni Vienna researchers performed molecular and spatial analyses at the single-cell level, revealing three distinct subtypes of CAFs: myofibroblast-like RGS5+ CAFs, matrix CAFs (mCAFs), and immunomodulatory CAFs (iCAFs). Notably, the distribution of these subtypes correlates with tumor aggressiveness, highlighting their potential as targets for new therapies.
Among these subtypes, mCAFs—typically found in less aggressive tumors—focus on producing extracellular matrix proteins that can create a barrier, impeding immune cell infiltration. In contrast, iCAFs, which are prevalent in more aggressive skin cancers, secrete a variety of signaling molecules. These biomolecules play critical roles in recruiting and activating immune cells, thus potentially turning the tables in the tumor's favor.
The study’s lead researcher, Beate Lichtenberger, emphasizes the implications of their findings, stating, "Interestingly, we found that healthy fibroblasts can be transformed into iCAF-like cells when they interact with proteins released by skin cancer cells. This indicates a window of opportunity to exploit these transitions in developing targeted therapies."
The importance of these discoveries cannot be overstated. By focusing therapeutics on the specific CAF subtypes—especially the iCAFs that actively modulate immune responses—there lies an exciting potential to enhance the efficacy of skin cancer treatments. Lichtenberger notes, "Targeted therapies could not only bolster the immune response against tumors but also mitigate the likelihood of cancer cell spread, leading to better patient outcomes."
As scientists and clinicians delve deeper into the complexities of tumor microenvironments, these findings represent a significant leap forward in the quest for innovative and effective skin cancer treatments. The journey towards revolutionizing immunotherapy begins here, with the hopes of transforming how we confront one of the most prevalent cancers worldwide.



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)