Unraveling the Connection Between Human Evolution and Frontotemporal Dementia: Groundbreaking Study Reveals Shocking Links!
2024-10-11
Author: Jia
A groundbreaking study led by researchers at the University of California, San Francisco, has unveiled astonishing insights into frontotemporal dementia (FTD) — a condition that often interferes with our most essential human abilities like communication and emotional engagement. The team, headed by renowned scientists Lorenzo Pasquini and William Seeley, suggests that the very genes that propelled human evolutionary development may also increase susceptibility to this devastating neurological disorder.
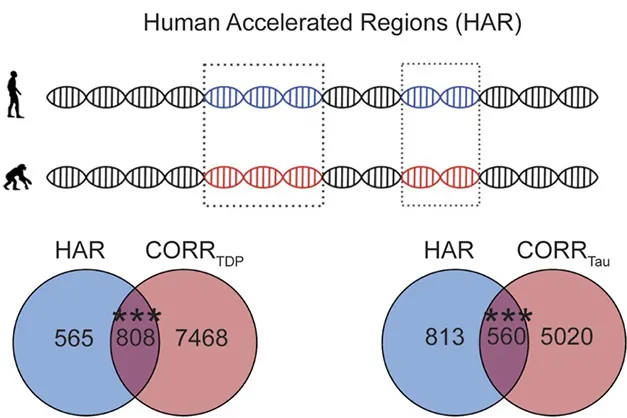
Published on September 3 in the journal Brain, the findings shed light on why specific forms of FTD target certain brain regions. The research synthesizes data from three pivotal areas: regional gene expression across the brain, human-accelerated regions (HARs), and the role of the DNA-binding protein TDP-43.
According to Seeley, the study's implications are profound: “We realized that the interplay between TDP-43 and human evolution might pose vulnerabilities — the very advancements that HARs bring could also become pitfalls.”
What Did the Study Involve?
The investigation analyzed brain donations from 164 participants, all contributed to UCSF's Neurodegenerative Disease Brain Bank. These individuals had undergone MRI scans during their lifetimes, and posthumous examinations revealed patterns of degeneration tied to common forms of FTD, particularly frontotemporal lobular degeneration (FTLD), marked by aggregations of either TDP-43 or tau proteins. The researchers identified five distinct FTLD subtypes each with unique degeneration patterns originating from different brain regions.
Using the Allen Human Brain Atlas as a reference, the team mapped gene expression across 273 brain regions. They discovered that genes correlated with brain atrophy in FTLD subtypes overlapped significantly with HAR genes — evidence that these evolutionarily altered genes could leave the human brain more prone to FTD.
Intriguingly, differences in gene expression also hinted at fundamental reasons why other primates experience lesser neurodegenerative issues. The researchers raise questions about the trade-offs of evolution — that while HAR genes have been associated with advanced cognitive functions, they may simultaneously set the stage for specific diseases.
The Mystery of Cryptic Splicing and TDP-43
The research did not stop at identifying atrophy-associated genes. It delved into how TDP-43 dysfunction could lead to mis-spliced genes, forming potentially toxic proteins. Analyzing 257 genes linked to this cryptic splicing, Pasquini found a significant overlap with genes tied to FTLD-TDP. This suggests the loss of TDP-43’s regulatory power may significantly contribute to FTD pathogenesis.
As the scientists look to the future, they are narrowing their focus to further investigate the role of 37 specific HAR genes that are susceptible to cryptic splicing and correlated with at least one subtype of FTLD-TDP. “These genes are the real treasures of our research,” Seeley emphasized, indicating plans to explore tissue studies to deepen understanding.
Expert Opinions and Next Steps
The study has garnered attention from experts in the field. Lary Walker, professor emeritus at Emory University, commented on the importance of this research, emphasizing that identifying why certain cells are more vulnerable could lead to novel therapeutic targets. However, caution was voiced by Peter Nelson from the University of Kentucky, who highlighted limitations in the datasets but praised the ambitious approach of examining multiple genes in the context of complex neurodegenerative diseases.
As researchers gear up for deeper investigations, the implications of this study resonate broadly — offering not only a glimpse into the genetic underpinnings of frontotemporal dementia but also challenging our understanding of human evolution's potential costs. With hopes pinned on these findings, we may soon be unlocking much-needed pathways for both treatment and preventive actions for FTD.
Stay tuned, as science continues to push boundaries in understanding the intricate links between evolution and health!


 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)