Unraveling the Secrets of Antibiotic-Resistant Bacteria: New Study Reveals Insights on S. pneumoniae Capsules
2025-03-24
Author: Yu
Introduction
Antibiotic-resistant bacteria have emerged as a formidable public health challenge, and understanding their biology is crucial for developing innovative strategies to combat this rising threat. A recent study published by researchers from the National University of Singapore (NUS Medicine) sheds light on the mechanisms that enable the bacterium *Streptococcus pneumoniae* to form its protective capsules—key components in its ability to evade the human immune system.
Background on *Streptococcus pneumoniae*
*Streptococcus pneumoniae*, commonly residing in the upper respiratory tract, is typically harmless in some individuals but poses grave risks to vulnerable populations, such as young children, the elderly, and those with weakened immune systems. This bacterium is responsible for severe diseases, including pneumonia and meningitis, which can be life-threatening.
Role of Protective Capsules
The protective capsule of *S. pneumoniae* acts as a shield, effectively warding off immune responses and facilitating the bacterium's survival and proliferation within the host. Researchers at NUS Medicine focused on these protective capsules, discovering how the bacterium constructs them through a series of complex transporter proteins.
Research Insights
Lead researcher Assistant Professor Chris Sham Lok-To emphasized the significance of understanding capsule synthesis as essential in combating pneumococcal infections. "By investigating how capsule transporters select their substrates, we can pave the way for new research avenues in bacterial evolution, antibiotic resistance, and vaccine development," he stated.
Decoding Capsule Transporters
The team’s findings detailed in *Science Advances* identified the Multidrug/Oligosaccharidyl-lipid/Polysaccharide (MOP) transporter family, which plays a critical role in moving sugar building blocks from within the bacterial cell to the capsule surface. Notably, this transport system is not just vital for the bacterium's growth but also has potential implications in glycoengineering—modifying sugar structures for drug development and enhancing biomaterials.
Research Methodology
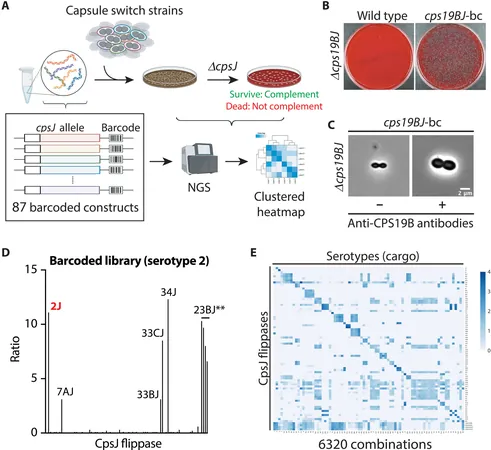
Researchers utilized a novel large-scale approach to assess more than 6,000 combinations of transporters and sugar building blocks. By inserting 80 different gene variants of transporters into 79 *Streptococcus pneumoniae* strains, they could track the genetic codes of each transporter, identifying which successfully facilitated capsule formation.
The Three Types of Transporters
Through their rigorous analysis, the researchers classified the transporters into three categories based on their selectivity: 1. **Strictly Specific Transporters**: These transporters operate solely with their designated sugar blocks, ensuring precision but limiting adaptability. 2. **Type-Specific Transporters**: These can accommodate sugars with shared features, enabling some interchangeability within similar capsule types. 3. **Relaxed Specificity Transporters**: These are capable of handling a broad range of sugars, but this flexibility can sometimes lead to negative consequences, such as transporting incomplete sugars that disrupt critical cellular processes.
Drawbacks of Transporter Specificity
Dr. Chua Wan Zhen, the first author, highlighted that the drawback of relaxed specificity transporters could hinder bacterial growth due to the accumulation of incomplete sugar precursors, which interfere with cell wall construction and can even lead to bacterial death.
Future Research Directions
The study’s findings underscore how subtle genetic modifications in transporter proteins can influence bacterial adaptability and virulence. This newfound understanding opens the door for innovative approaches to tackle bacterial infections and possibly engineer advantageous sugar-based materials.
Conclusion and Implications
Future studies will aim to pinpoint the specific amino acid residues responsible for interactions between transporters and substrates. This line of research holds promise for optimizing transporter specificity, potentially leading to groundbreaking advancements in industrial applications and healthcare solutions.
As antibiotic resistance continues to escalate, this study could provide the foundational insights necessary for developing new therapeutic strategies and vaccines, ultimately aiding in the global fight against antibiotic-resistant infections.

 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)