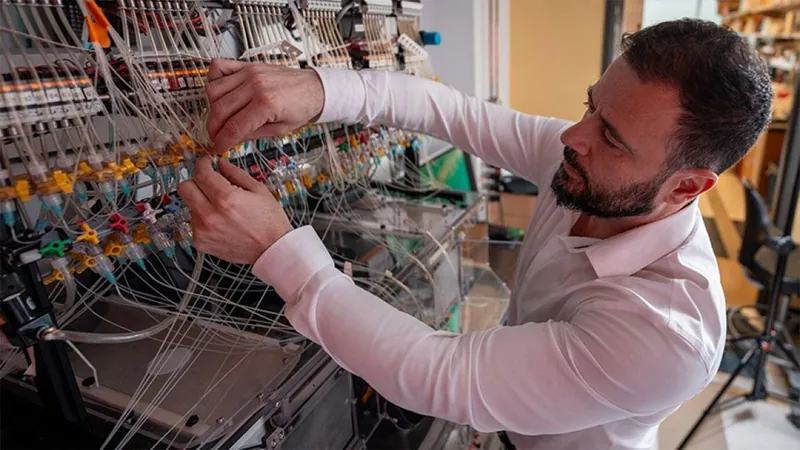
Unveiling the Secrets of Immune Memory: How Macrophages Adapt to Prior Infections
2025-03-20
Author: Arjun
Scientists have long credited antibodies with the remarkable ability of the human immune system to remember past infections. However, groundbreaking research from the University of Chicago's Pritzker School of Molecular Engineering has revealed an unexpected player in this phenomenon: macrophages, a vital type of white blood cell.
These researchers found that macrophages can significantly change their signaling behavior right after an infection occurs. This “short-term memory” influences how these cells tackle subsequent infections or react to immune signals. In some instances, macrophages exhibit tolerance and become less responsive, but in other cases, they bolster their immune response, providing a dual mechanism for adapting to the body's needs.
Published in the esteemed journal, Cell Systems, these findings could reshape our understanding of immune system functionality and pave the way for innovative treatments aimed at controlling macrophage activities to combat infections or autoimmune conditions.
“Macrophages can adjust their responses based on their exposures,” explained Professor Savas Tay from UChicago PME, who led the study. “Understanding the dynamics of inflammatory signals can inspire more advanced cell therapies that harness this adaptability to improve immune control.”
Redefining What We Know About Immune Memory
The human immune system has two principal defenses. The innate immune system acts swiftly and generally, while the adaptive immune response is slower and geared towards specific invaders. Traditionally, it's been understood that innate immunity, which includes macrophages, does not exhibit memory or adaptability akin to that of adaptive immunity.
Andrew Wang, an M.D./Ph.D. student and the primary author, noted that recent studies have suggested that macrophages might indeed vary their responses based on past encounters. Intrigued by this revelation, he and his team explored whether these immune cells undergo changes over time.
Utilizing a state-of-the-art, high-throughput microfluidics platform, the researchers examined how 80 distinct conditions influenced macrophage activity in response to different bacterial and viral agents. Their analysis uncovered a fascinating “priming” effect whereby macrophages became more reactive to future threats. However, they also found that exposure to certain inflammatory signals could induce tolerance, causing these cells to become less responsive during subsequent encounters.
The Complexity of Immune Signaling
Wang highlighted the intricacy of immune signaling, which produces diverse effects based on a variety of factors. Notably, the relationship between the type of pathogen and its impact on immune signaling was not straightforward. Generally, prolonged or higher doses of exposure to pathogens tended to increase tolerance, potentially serving as a protective mechanism to avoid overactive immune responses. Conversely, brief interactions or lower doses often led to enhanced readiness in macrophages.
In a striking finding, the researchers isolated macrophages from mice suffering from sepsis, a serious condition characterized by extensive inflammation often triggered by severe infections. These macrophages exhibited reduced immune responses, shedding light on the vulnerability of sepsis patients to secondary infections. This opens the door to potential interventions aimed at reviving macrophage activity or mitigating tolerance, presenting promising avenues for sepsis treatment.
Predicting Immune Reactions
The team also discovered how macrophage activity fluctuated in tandem with changes in a crucial immune regulator known as nuclear factor kappa-B (NF-κB). They found that exposure to inflammatory signals modulated both NF-κB activation and its entry into the nucleus over time, altering the accessibility of specific DNA regions necessary for inflammatory gene activation.
Using this information, the researchers developed a machine-learning model that predicts how macrophages will respond to new inflammatory signals based on their previous experiences.
“What we’re uncovering is how a pathogen can influence a macrophage to reach a new steady state,” Wang stated. “This insight not only enriches our understanding of inflammatory signaling complexity but also carries practical implications for developing targeted therapies.”
The Future of Immune Research
As scientists delve deeper into the adaptive capabilities of macrophages, the potential for innovative therapies to enhance immune responses grows brighter. This remarkable discovery underscores the immune system's complexity and adaptability, challenging long-held beliefs and offering hope for more effective treatments for infections and autoimmune diseases. Stay tuned as we continue to explore the evolving landscape of immunology!




 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)