WHO Greenlights Bavarian Nordic's Mpox Vaccine for Teens: A New Shield Against Outbreaks!
2024-10-14
Author: Rajesh
WHO Approves Jynneos Vaccine for Adolescents
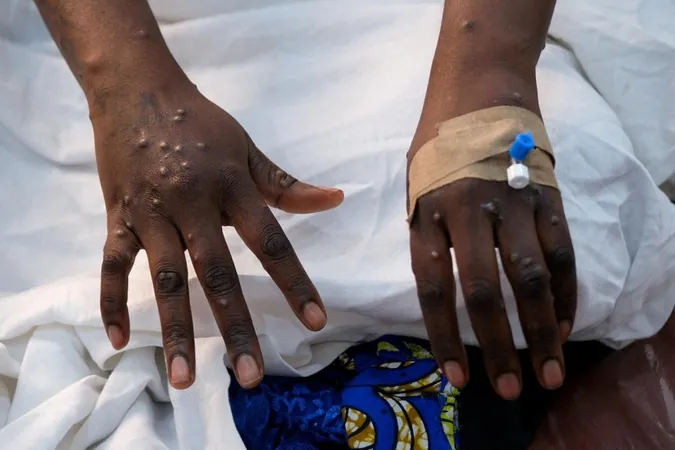
In a significant move that strengthens defenses against the alarming spread of mpox, the World Health Organization (WHO) announced on Monday the approval of Bavarian Nordic's Jynneos vaccine for adolescents aged 12 to 17. This age group has been identified as particularly vulnerable in light of recent outbreaks, which have raised global health concerns.
Prequalification and Global Health Emergency
The WHO granted this prequalification on October 8, enhancing the ability of affected regions to secure access to this much-needed vaccine. In August, the WHO had declared mpox a global public health emergency for the second time in two years following a troubling emergence of the virus that has spread from the Democratic Republic of Congo to surrounding nations.
Previous Approvals and Vaccine Importance
Prior to this, in September, the WHO had already approved the Jynneos vaccine for adults, which was a monumental step in providing resources to countries hit hard by mpox. The vaccine is essential as the mpox virus can cause severe flu-like symptoms, including painful skin lesions filled with pus, particularly affecting children and individuals with weakened immune systems.
Synchronized Global Efforts on Immunization
The recent WHO decision aligns with the European Union’s approval of the vaccine for young adolescents earlier in September. This synchronized effort highlights a growing consensus on the necessity of immunizing younger populations against mpox.
Future Trials for Younger Children
In an exciting development, the Danish biotech firm is preparing to launch a clinical trial to evaluate the vaccine's safety specifically in children aged two to 12 years. Funded in part by the Coalition for Epidemic Preparedness Innovations, this trial is expected to initiate in October, aiming to broaden the vaccine's applicability.
Regulatory Approvals in the United States and Japan
Meanwhile, in the United States, the Food and Drug Administration (FDA) has approved Bavarian Nordic’s vaccine for adults aged 18 and older but previously granted Emergency Use Authorization for its administration in adolescents during the mpox outbreak of 2022. As for Japan, another mpox vaccine, LC16, manufactured by KM Biologics, is already available for children, although its administration requires a specialized type of needle.
Conclusion and Next Steps
As the global community continues to grapple with this infection, the approval of the Jynneos vaccine marks a crucial step forward in safeguarding young populations against mpox. Stay tuned for more updates on this developing story, as the race to protect public health accelerates!



 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)