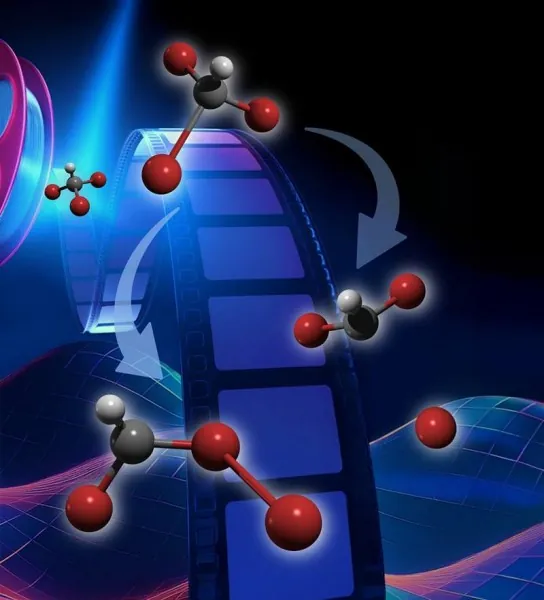
Bromoform Molecules Unveil Mysteries of Light Interaction: A Major Breakthrough!
2024-11-19
Author: Jacob
The Science Behind the Discovery
Bromoform (CHBr3) is not just an ordinary molecule; it's a potent naturally occurring compound known for its role in ozone depletion in our atmosphere. When exposed to ultraviolet (UV) light, bromoform undergoes fascinating transformations—specifically, it can either break one of its bromine atoms away (a process called "bond dissociation") or rearrange its atomic structure without losing its molecular integrity (known as "isomerization").
Despite numerous studies, the interplay between these processes has remained elusive due to the astonishingly rapid speeds at which they occur—happening in mere picoseconds (trillionths of a second). New experiments have uncovered that approximately 60% of bromoform molecules interacting with UV light favor isomerization over bond dissociation. This pivotal finding opens new avenues for understanding the critical role isomerization plays in bromoform's interactions with light.
The Significant Implications of This Research
For the very first time, researchers have quantitively assessed how bromoform molecules respond to light, distinguishing between those that experience direct bond dissociation and those that undergo isomerization. This represents a monumental leap toward comprehending how bromoform isomers—the various configurations of the bromoform molecule—are formed. Historically, while these isomers were theorized, empirical data concerning their formation rates had been lacking.
This groundbreaking research challenges established theories in physical chemistry, which were unable to adequately explain the behavior of bromoform in all situations. These new findings not only corroborate some existing predictions but also contradict others, prompting a reevaluation of our understanding of bromoform's chemistry and potentially paving the way for new theoretical models.
Unlocking the Ultrahigh-Speed Processes
The research team leveraged the advanced relativistic ultrafast electron diffraction (UED) instrument at the SLAC National Accelerator Laboratory, which allowed them to obtain crucial insights into the ultrafast dynamics of bromoform’s reactions. This state-of-the-art instrument, part of the Linac Coherent Light Source—an esteemed user facility within the Department of Energy's Office of Science—enables observation of molecular motions in real-time.
According to the findings, approximately 60% of bromoform molecules isomerize in the first fifth of a picosecond, and these isomers remain stable for the duration of the experiment, spanning 1.1 picoseconds. Current theoretical frameworks have yet to predict this combination of quick formation and stability, indicating a significant gap in our understanding that future studies will need to address.
The Research's Broader Impact and Future Directions
This important work was financed by the Department of Energy (DOE), specifically the Office of Science, and its findings are anticipated to propel further investigations into molecular photochemistry. Understanding bromoform's reactions can have wider implications, especially concerning environmental science and the impact of chemical compounds on ozone depletion and climate change.
As researchers delve deeper into the complexities of molecular behavior under UV exposure, we may soon uncover more secrets held by nature, one molecule at a time.
Stay tuned, as this discovery is set to challenge the boundaries of chemical science and may even redefine how we think about atmospheric interactions in our ever-changing world!






 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)