Game-Changer in Biochemistry: New Enzyme Subclass Unveiled for Antimicrobial Lanthipeptide Synthesis
2024-11-29
Author: Noah
Introduction
In a groundbreaking discovery, biochemists at the National University of Singapore (NUS) have identified a new subclass of trifunctional enzymes in gram-positive bacteria that is essential for the biosynthesis of a powerful antimicrobial compound known as lanthipeptides.
Significance of Lanthipeptides
These remarkable biomolecules, synthesized by gram-positive bacteria in response to environmental stressors, are garnering attention due to their potent antibacterial, antifungal, and antiviral properties. With the rising global threat of antibiotic resistance, the potential of lanthipeptides as a source for innovative drug development has never been more critical.
Challenges in Understanding Lanthipeptide Biosynthesis
Despite their significant therapeutic implications, the intricacies of how these peptides undergo vital post-translational modifications—a process crucial for their effectiveness—had remained shrouded in mystery until now.
Research Methodology
The research team, spearheaded by Assistant Professor Luo Min from the Department of Biological Sciences at NUS, utilized advanced methods including bioinformatics, cryo-electron microscopy, and functional assays to delve deep into this complex biochemical process. Their efforts led to the characterization of a unique subclass of enzymes responsible for modifying lanthipeptides.
Discoveries Made
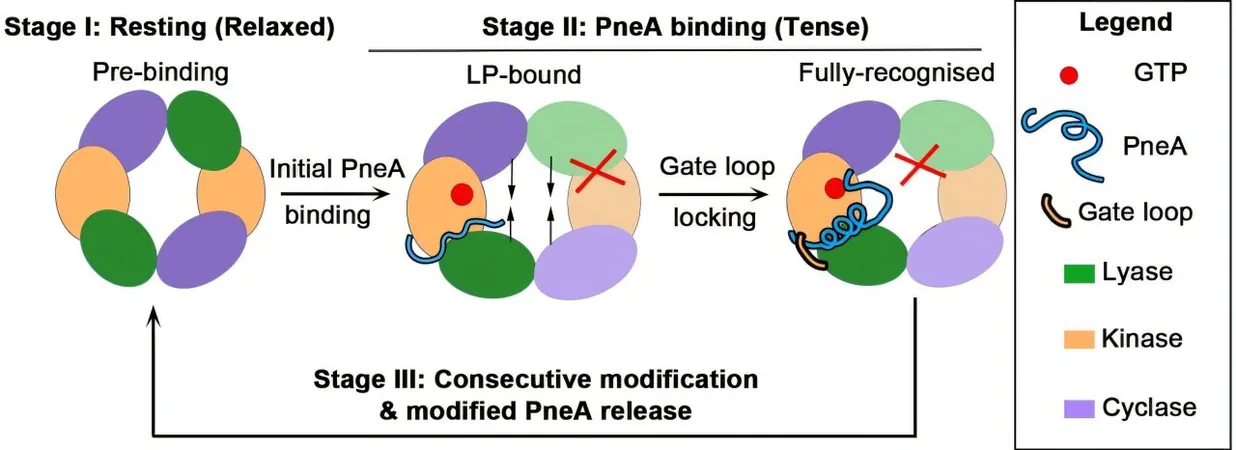
Among their most notable findings was the discovery of the first homodimeric lanthipeptide modification enzyme, identifiable as PneKC, derived from the bacterium *Streptococcus pneumoniae*. Structural analysis unveiled an intriguing component dubbed the "dimerization hotspot," where two segments of the enzyme converge to form a functional dimer. Interestingly, when this specific region was altered, the enzyme was incapable of forming its necessary dimeric structure, underscoring the importance of this architectural feature.
Mechanism of Action
Published in the reputable journal Nature Communications, the research outlines how the dimeric enzymes exhibit a self-regulatory behavior through a phenomenon known as negative cooperativity. When a substrate binds to one half of the dimer, it instigates a conformational change that is transferred to the other half, effectively modulating the active site’s readiness.
Further Findings
Additionally, the researchers uncovered the initial phases of how the peptides are recognized by these enzymes and identified a set of previously unknown catalytic residues located in the enzyme's active site. These specific amino acids are vital for executing the final modification step, particularly the cyclization process—an essential transformation that allows lanthipeptides to achieve their functional form.
Implications for Drug Development
This discovery fills a crucial gap in our understanding of lanthipeptide biosynthesis, paving the way for new strategies in drug development aimed at combating resistant strains of bacteria.
Future Research Directions
Moving forward, Ms. Yifan Li, a Ph.D. candidate and lead author of the study, is now dedicated to exploring the subsequent stages of lanthipeptide maturation and pharmacodynamics. Her work aims to unlock further potential therapeutic applications for these newly categorized lanthipeptides.
Conclusion
Professor Luo remarked on the study's significance, stating, "Our research has greatly enhanced our understanding of the dynamic processes involved in peptide recognition and coordination within lanthipeptide modification enzymes. Given the escalating need for new antimicrobial agents, these insights will be pivotal in the quest for novel lanthipeptides with therapeutic efficacy."
Looking Ahead
Stay tuned to find out how this revelation could revolutionize the future of medicine in the fight against some of the world's toughest pathogens!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)