Groundbreaking Discovery Reveals How a Cellular Supercomplex Bridges mRNA Translation and Decay!
2024-10-10
Author: Amelia
Groundbreaking Discovery Reveals How a Cellular Supercomplex Bridges mRNA Translation and Decay!
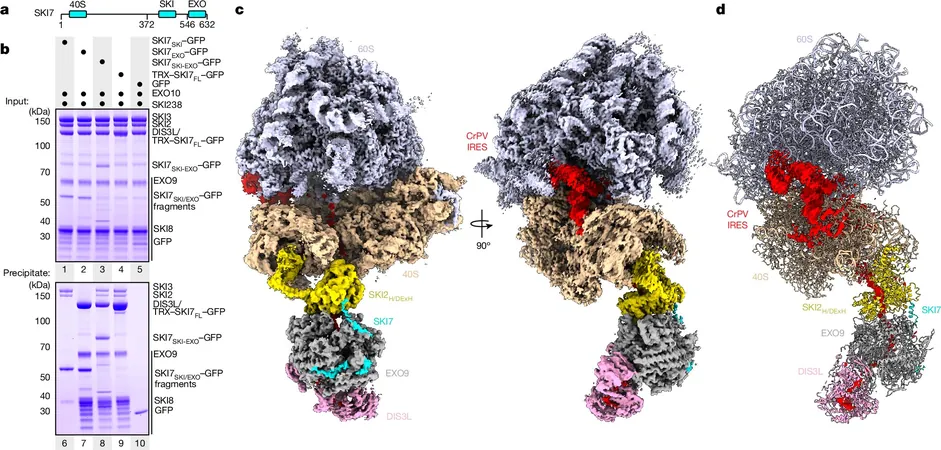
In a remarkable development that could reshape our understanding of cellular biology, researchers led by Director Elena Conti at the Max Planck Institute of Biochemistry have unveiled high-resolution structural data illustrating how messenger RNA (mRNA) translation and decay are interconnected through a supercomplex. This groundbreaking structure comprises three key components: the ribosome, the SKI complex, and the exosome, all of which play vital roles in the cell's quality control processes.
For years, scientists have understood the individual functions of these complexes, but how they interact remained a mystery. Ribosomes, commonly known as the cell’s protein factories, are crucial for translating mRNA into proteins, linking amino acids in a precise order based on the RNA sequence. This translation is pivotal for the synthesis of proteins that are essential for various cellular functions.
When mRNA is no longer needed or is found to be defective, it must be carefully degraded. This is where the SKI complex comes into play. Acting like a molecular courier, it transports faulty or surplus mRNA to the exosome, a powerhouse that functions similarly to a shredder, ensuring that unwanted RNA is effectively dismantled.
The innovative research team, equipped with advanced microscopy techniques, found that these complexes do not operate in isolation. Instead, they form a supercomplex that facilitates the coupling of translation and degradation, allowing cells to maintain a high level of quality control over their genetic information. Previous studies hinted at collaboration between the SKI complex and exosome; however, the identification of a supercomplex dramatically enhances our understanding of RNA fate determination.
Importantly, the research also focused on circumstances where mRNA is damaged. In typical situations, multiple ribosomes can attach to a single mRNA strand but when errors occur, ribosomes may collide. By simulating these collisions, the team demonstrated that the damaged mRNA would attract the SKI complex, targeting it for breakdown by the exosome.
In a fascinating analogy, this process resembles a quality control check on an industrial production line. The SKI complex, upon detecting an error during translation, can unwind the mRNA and transport it to the exosome for degradation. A key player in this operation is another protein, SKI7, which connects the SKI complex to the exosome, emphasizing the intricate dance of molecular interaction at play.
The findings from this pivotal study, published in the journal Nature, shed light on an elaborate network of molecular machinery that is critical for regulating gene expression. As researchers continue to investigate these systems, the implications for biotechnology and therapeutic applications are enormous, hinting at potential breakthroughs in understanding diseases where RNA metabolism goes awry.
Stay tuned as we delve deeper into the implications of this research and what it means for the future of molecular biology! Could this lead to innovative treatments for diseases linked to RNA malfunction? Only time will tell!








 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)