Groundbreaking Discovery Unveils Mechanism of Cellular Recycling: What You Need to Know!
2024-12-09
Author: Charlotte
Introduction
In a significant advancement for cell biology, a recent study published in eLife has revealed a critical mechanism behind how cells manage and recycle their materials, focusing on endosome maturation. Led by prominent researchers Dr. Ding Mei and Dr. He Kangmin from the Institute of Genetics and Developmental Biology at the Chinese Academy of Sciences, this research uncovers essential processes vital for cell health and overall functionality.
Endosomes: The Sorting Stations of Cells
Endosomes act as vital 'sorting stations' within our cells, where they handle various substances, including solutes, receptors, lipids, and pathogens. This sorting process is pivotal, as early endosomes determine which materials will be recycled back to the cell surface and which ones are destined for degradation. Materials earmarked for disposal transition to late endosomes and ultimately into lysosomes, where they undergo complete breakdown.
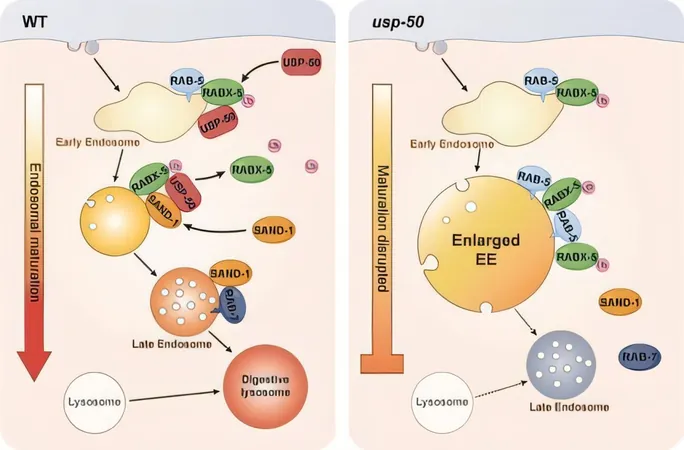
The Transition from Early to Late Endosomes
A fascinating aspect of this process is the transition from early endosomes to late endosomes, which involves a precise 'molecular handoff.' The early phase is marked by the presence of the Rab5 protein, while the transition to the late phase sees this protein being replaced by Rab7. However, until now, the specific details of how this switch occurs remained largely unknown.
Discovering a Crucial Gene: usp-50
In their investigation, Dr. Ding's team utilized the model organism Caenorhabditis elegans to pinpoint a critical gene known as usp-50. This gene encodes a deubiquitinating enzyme, essential for regulating endosome maturation by removing ubiquitin tags from specific proteins—which is crucial for the Rab5-to-Rab7 transition.
Impact of Gene Absence on Endosomes
Their findings revealed alarming results: when the usp-50 gene is absent, early endosomes grow excessively large, the number of late endosomes dwindles, and the formation of functioning lysosomes is negatively impacted. These disruptions are detrimental to the cell's ability to handle its material effectively.
Similar Mechanism in Humans
Furthermore, the researchers demonstrated that the mechanism identified in C. elegans is mirrored in human cells through the protein USP8, which serves a function analogous to USP-50. USP8 interacts with Rabex5, a crucial activator for Rab5, and removes ubiquitin tags from it. This critical de-ubiquitination process allows Rabex5 to disengage from early endosomes, facilitating the subsequent activation of Rab7 by proteins like SAND-1/Mon1, thereby ensuring effective endosome maturation.
Implications for Medicine
What does this mean for the future of medicine? This breakthrough not only enhances our understanding of cellular biology but has profound implications for developing targeted therapies for a range of diseases linked to defects in cellular material management. Notably, abnormalities in USP8 have been associated with Cushing's syndrome and various cancers—conditions where USP8 operates in overdrive or dysfunction.
Conclusion
'This research significantly advances our understanding of cell biology and opens up remarkable possibilities for addressing diseases caused by errors in cellular waste management,' states Dr. Ding. As scientists dive deeper into the implications of these findings, the potential for breakthroughs in treating disorders rooted in cellular dysfunction becomes increasingly tangible. Stay tuned for more updates on how this research could revolutionize medical approaches in the coming years!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)