Revolutionary Breakthrough: Sustainable Method Electrosynthesizes Key Chemical for Synthetic Rubber Production!
2024-11-23
Author: William
Introduction
In an exciting development for the chemical industry, a talented team of chemists from the National University of Singapore (NUS) has unveiled a groundbreaking sustainable method to electrosynthesize 1,3-butadiene, a crucial feedstock for synthetic rubber production, from acetylene. This innovative approach promises to drastically reduce the energy requirements and environmental impact normally associated with producing multi-carbon molecules.
Significance of Electrification
As the world increasingly calls for greener solutions, electrification has emerged as a transformative strategy. By harnessing renewable electricity to convert simple feedstocks like water and carbon dioxide (CO2) into valuable chemicals and fuels, scientists are paving the way for a more sustainable future. Among these vital target molecules is 1,3-butadiene, which, despite its essential role, is currently produced in large quantities—over 18 million tons annually—only as a minor by-product from the energy-intensive cracking processes of naphtha or ethane.
Research Findings
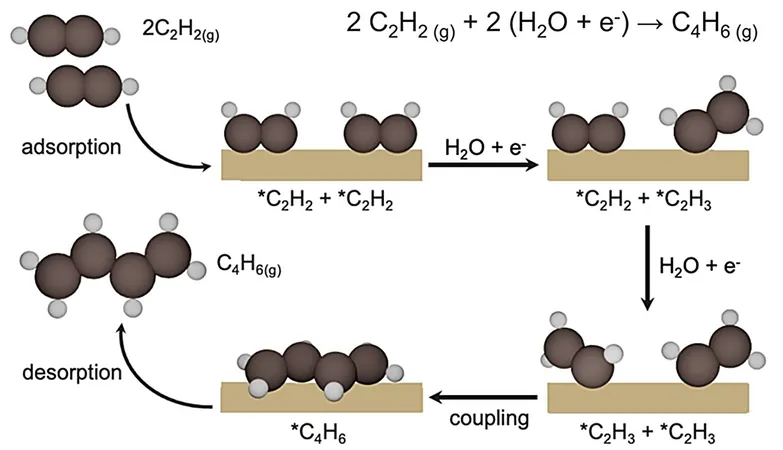
Leading the research team, Associate Professor Yeo Boon Siang, Jason, from the Department of Chemistry at NUS, discovered that modified copper catalysts, enhanced with iodide anions, are highly effective in converting acetylene to 1,3-butadiene. The impressive results of this study were published in the prestigious journal Nature Catalysis.
Catalytic Performance
In laboratory tests, the catalysts demonstrated a remarkable Faradaic efficiency of 93% at -0.85 V versus the Standard Hydrogen Electrode (SHE) and achieved a partial current density of -75 mA cm-2 at -1.0 V versus SHE. This partial current density, a critical measure of catalytic activity, was at least 20 times greater than results reported in earlier studies, marking a significant leap forward in the field.
Collaboration and Team Effort
This collaborative effort also included notable researchers such as Dr. Federico Calle-Vallejo from Spain's Basque Foundation for Science and the University of the Basque Country, alongside key contributors from the University of Barcelona, and Shell Global Solutions International B.V., demonstrating a robust international partnership aimed at advancing sustainability in chemical production.
Catalyst Characterization
In-depth characterization of the catalyst was conducted using advanced analytical techniques and computational simulations, unveiling that iodide modulates the stability of certain copper sites (Cuδ+–Cu0) crucial for facilitating the carbon-carbon (C–C) coupling of C2H3 intermediates, ultimately leading to the synthesis of 1,3-butadiene.
Future Plans
Reflecting on the collaboration, Prof. Yeo expressed, 'This work is the result of intense teamwork among experimentalists, theoreticians, and our industrial partners to uncover how essential chemicals, like 1,3-butadiene, can be produced more sustainably.' Looking ahead, the research team is eager to build upon their findings and is already planning to develop catalysts that will enable the coupling of acetylene into longer-chain hydrocarbons, potentially transforming them into sustainable aviation fuels.
Conclusion
This remarkable breakthrough not only sets the stage for rapid advancements in synthetic rubber materials but also ignites hope for a greener future by reducing our reliance on traditional petrochemical processes. The implications of this research are vast, and it could reshape industries from automotive to aerospace while addressing urgent environmental concerns. Stay tuned for more updates as this saga of sustainability unfolds!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)
 الإمارات العربية المتحدة (AR)
الإمارات العربية المتحدة (AR)