Unveiling the Secrets of Hydrogenation: The Journey of Interstellar Polyynes
2025-01-12
Author: Charlotte
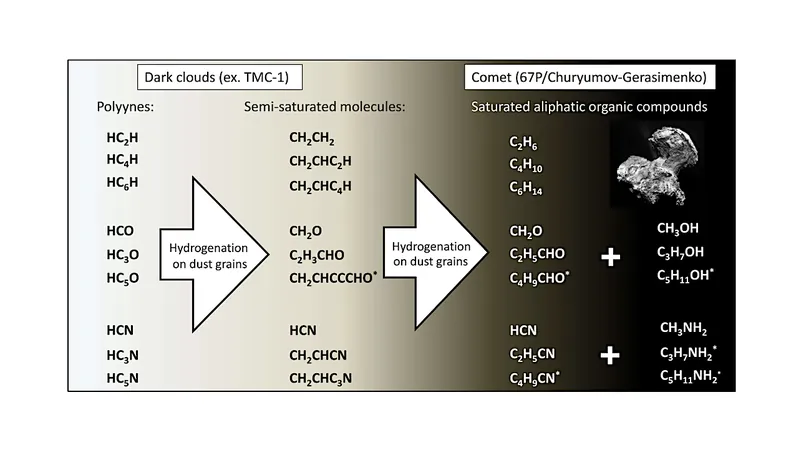
In an exciting advancement for astrophysics, highly unsaturated carbon chains, particularly polyynes, have been identified across various astronomical regions and planetary systems. This remarkable discovery has sparked a significant increase in research, especially following the groundbreaking findings from the QUIJOTE survey of the TMC-1 molecular cloud, which has led to a "boom" in the detection of these elusive carbon structures.
The Rosetta mission further expanded our understanding by revealing fully saturated hydrocarbons such as propane (C3H8), butane (C4H10), pentane (C5H12), and, under specific conditions, hexane (C6H14) and heptane (C7H16) emanating from comet 67P/Churyumov-Gerasimenko. These discoveries were notably linked to dust-rich events, emphasizing the complex chemistry at play in our solar system.
Moreover, a detailed analysis of samples collected from asteroid Ryugu by Japan's Hayabusa2 mission unveiled the presence of long saturated aliphatic chains within Ryugu's organic matter, strengthening the ties between celestial bodies and organic chemistry.
A crucial piece of the puzzle lies in understanding the surface chemistry of unsaturated carbon chains under conditions akin to those found in molecular clouds, which could establish a connection among these otherwise disparate observations. However, until now, experimental investigations validating these ideas have been sorely lacking.
In response, a recent study has undertaken to experimentally verify the hydrogenation processes of polyynes, focusing on compounds with the formula C2nH2 (with n greater than 1) under ultra-high vacuum conditions at a chilling temperature of 10 K.
The innovative two-step experimental approach involved initially creating a thin layer of acetylene (C2H2) ice, which was then subjected to UV-photon irradiation (wavelengths ≥ 121 nm). This process led to the partial conversion of acetylene into larger polyynes such as C4H2 and C6H2. Following this, the ice was exposed to hydrogen atoms, aiming to verify the formation of various saturated hydrocarbons.
The findings shed light on the formation of ethane (C2H6), which had been studied previously, alongside the successful identification of larger alkanes, including butane (C4H10) and tentatively hexane (C6H14). A qualitative analysis of the kinetic data further suggests that the hydrogenation process of HCCH (acetylene) and HCCCCH (a longer-chain alkyne) occurs at rates that are comparable under the specified temperature conditions.
This research not only enhances our understanding of organic chemistry in space but may also shed light on the origins of complex molecules essential for life as we know it. The implications of this study could revolutionize our understanding of how organic compounds form in the universe! Stay tuned as we continue to unravel the mysteries of cosmic chemistry!









 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 Česko (CS)
Česko (CS)
 대한민국 (KO)
대한민국 (KO)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)