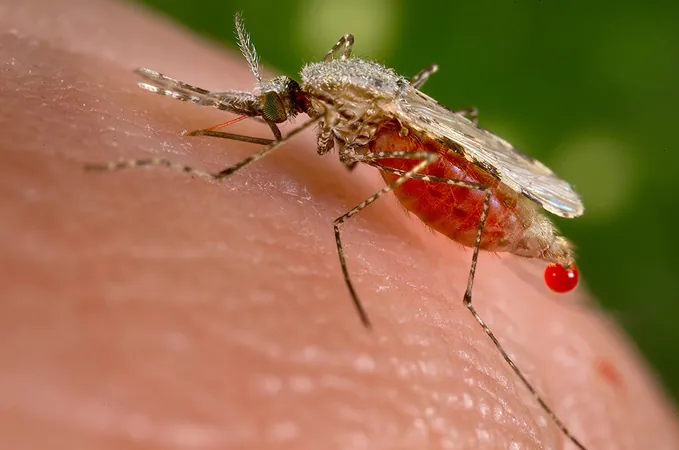
Groundbreaking Malaria Vaccine Candidates Show Promise in Clinical Trials
2024-12-11
Author: Ming
Groundbreaking Malaria Vaccine Candidates Show Promise in Clinical Trials
In a significant breakthrough in the fight against malaria, a new vaccine candidate known as RH5.1/Matrix-MTM has demonstrated effective protection against the blood-stage of the disease in clinical trials. This is a landmark achievement as it is the first vaccine to target this critical stage of malaria caused by the *Plasmodium falciparum* parasite, which remains a leading cause of mortality among children under five in numerous African regions.
The findings stem from a clinical trial conducted by researchers at the University of Oxford in conjunction with experts from the Clinical Research Unit of Nanoro (CRUN) in Burkina Faso, London's School of Hygiene and Tropical Medicine, and the National Institutes of Health (NIH) in the United States. The study, supported by global partners such as the Serum Institute of India, Novavax, and ExpreS2ion Biotechnologies, sought to evaluate the safety and efficacy of the RH5.1/Matrix-MTM vaccine.
In this 2023 study, over 360 children aged 5 to 17 months were enrolled and divided into two groups: one group received the malaria vaccine while the control group was administered a rabies vaccine. Importantly, the trial was designed as a double-blind study, ensuring that participants and researchers did not know which vaccine was being administered.
Published in *The Lancet Infectious Diseases*, the trial results revealed that RH5.1/Matrix-MTM was not only well-tolerated but also extremely effective. Children receiving the vaccine exhibited elevated antibody levels against the malaria parasite, particularly those receiving their doses at longer intervals. The vaccine displayed a promising 55% effectiveness in preventing clinical malaria within a six-month evaluation period. More impressively, it showed over 80% efficacy against severe malaria cases, characterized by high parasite levels in the bloodstream.
Professor Angela Minassian, a leading expert on the clinical blood-stage malaria vaccine program at Oxford, emphasized the importance of this vaccine in reducing severe malaria cases and related deaths. Unlike existing vaccines such as RTS,S/AS01 and R21/Matrix-MTM that focus on the liver stage of the parasite, RH5.1/Matrix-MTM targets the blood stage where complications like anemia and organ failure manifest. This versatile approach could be pivotal in the arsenal against malaria.
Halidou Tinto, a prominent parasitology professor in Burkina Faso, remarked on the vaccine's potential to improve the health and educational outcomes for children, highlighting the correlation between frequent malaria infections and childhood development issues.
The study is ultimately paving the way for subsequent trials, with hopes for a combined vaccination strategy that enhances efficacy. Professor Simon Draper, who has been at the forefront of developing the RH5.1 vaccine, hailed the results as a major milestone for malaria research given the historical challenges of creating an effective blood-stage malaria vaccine.
With such advancements, the future looks brighter in the ongoing battle against malaria, a disease that has claimed millions of lives and continues to pose a significant threat to populations, particularly in sub-Saharan Africa.
 Brasil (PT)
Brasil (PT)
 Canada (EN)
Canada (EN)
 Chile (ES)
Chile (ES)
 España (ES)
España (ES)
 France (FR)
France (FR)
 Hong Kong (EN)
Hong Kong (EN)
 Italia (IT)
Italia (IT)
 日本 (JA)
日本 (JA)
 Magyarország (HU)
Magyarország (HU)
 Norge (NO)
Norge (NO)
 Polska (PL)
Polska (PL)
 Schweiz (DE)
Schweiz (DE)
 Singapore (EN)
Singapore (EN)
 Sverige (SV)
Sverige (SV)
 Suomi (FI)
Suomi (FI)
 Türkiye (TR)
Türkiye (TR)